Iodoform
Synonym(s):CHI3;CHIT;chitinase 1 (chitotriosidase);Triiodomethane
- CAS NO.:75-47-8
- Empirical Formula: CHI3
- Molecular Weight: 393.73
- MDL number: MFCD00001069
- EINECS: 200-874-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
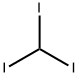
What is Iodoform?
Absorption
Iodoform is reported to be absorbed through denuded skin, wounds or mucous membranes [L2646].
Toxicity
Systemic intoxication and severe poisoning, mostly characterized by dermatitis, headache, somnolence, delirium, hallucinations, consciousness disturbances, coma, vomiting, CNS depression, tachycardia, and rapid feeble pulse, may result from excess absorption through wounds and abscesses, or ingestion of large quantities [L2646]. While there is no known antidote for iodoform overdose, supportive and symptomatic treatment is highly recommended [L2646].
Hepatocellular damage has been observed with iodoform toxicity, where production of both fatty liver and necrosis was seen, as well as lesions of liver membranous cellular components [L2646]. Iodoform induces hepatocellular injury and hepatic lesions in a similar manner to carbon tetrachloride, where cellular membranes may possibly be primary sites of action .
Description
Iodoform is a stable, pale yellow crystalline solid. It is volatile with a characteristic pungent, unpleasant and penetrating odour, but with a sweetish taste. It is incompatible with strong oxidising agents, reducing agents, lithium, and metallic salts such as mercuric oxide, silver nitrate, strong bases, calomel, and tannin. Reports indicate that earlier iodoform was in use as a disinfectant and as an antiseptic or dressing and healing of wounds and sores for pets. Iodoform is the active ingredient in many antiseptic or dressing powders used for dogs and cats to prevent infection.
Chemical properties
yellow solid with a characteristic pungent and
Chemical properties
Iodoform is a yellow or greenish-yellow powder or crystalline solid that is volatile with steam and contains 96.69% iodine. It has a very characteristic pungent odor. An odor threshold of 0.005 ppm has been reported.
The Uses of Iodoform
Iodoform has limited use as a chemical intermediate and for medicinal purposes as disinfectant and antiseptic and has been used in veterinary medicine.
The Uses of Iodoform
Triiodomethane is a crystalline compound commonly used as a disinfectant. It can be used in the process of removing heavy metals such as mercury from fuids.
The Uses of Iodoform
It is used as a topical anti-infective, applied to the skin.
Background
Iodoform is an organoiodine compound with the formula CHI3 and a tetrahedral molecular geometry. It is a relatively water-insoluble yellow solid that is chemically reactive in free-radical reactions . Due to its antimicrobial properties following topical administration, minimal levels of iodoform may be found in disinfectants and it is more primarily used for veterinary purposes. Iodoform has also been found in dental paste and root canal filling materials in combination with other intracanal medications due to its radiopacity . Since the beginning of the 20th century, iodoform has been commonly used as a healing and antiseptic dressing or powder for wounds and sores, however such clinical use to this date is limited. Iodoform is soluble in fatty acids and decomposes releasing iodine in nascent state (96,7% of iodine) when in contact with secretions or endodontic infections .
Indications
No approved therapeutic indications.
Definition
A yellow crystalline
compound made by warming ethanal with
an alkaline solution of an iodide:
CH3CHO + 3I– + 4OH– → CHI3 +
HCOO– + 3H2O
The reaction also occurs with all ketones of
general formula CH3COR (R is an alkyl
group) and with secondary alcohols
CH3CH(OH)R. Iodoform is used as a test
for such reactions (the iodoform reaction).
General Description
Bright yellow or yellow powder or crystals. Penetrating odor. Unctuous touch. Odor threshold 0.4 ppb.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Iodoform decomposes at high temperatures. Decomposes slowly in light at room temperature. Reacts violently with lithium. Is incompatible with mercuric oxide, calomel, silver nitrate, tannin, and balsam Peru. Is also incompatible with strong bases, strong oxidizing agents and magnesium. Vigorous reactions occur with acetone in the presence of solid potassium hydroxide or calcium hydroxide, hexamethylenetetramine at 352° F, mercury(I) fluoride and finely divided reduced silver.
Hazard
Irritant. Decomposes violently at 400F (204C).
Health Hazard
Iodoform causes central nervous system depression and damage to the kidneys, liver, and heart.
Fire Hazard
Literature sources indicate that Iodoform is nonflammable.
Biochem/physiol Actions
Iodoform is an iodine containing compound, which is used in antiseptic applications in the medical and veterinary medicine fields. Iodoform improves fermentation in the biomass ensilage conversion system by improving lactic acid production and inhibiting butyric fermentation.
Pharmacokinetics
Iodoform exhibits antibacterial activities after topical application. In a comparative study of wound dressing agents, iodoform gauze exerted an antibacterial effect 3 hours after the start of bacterial growth of E. coli and subsequently maintained the strong antibacterial effectiveness . A study demonstrated that direct and indirect exposure to high concentrations of iodoform induces a cytotoxic effect on cultures of macrophages and epithelial cells in vitro, while cell proliferation was enhanced at low concentrations of iodoform . This cytotoxic effect of iodoform in root canals may further lead to long-term local irritation to follicles of permanent successors and formation of cyst-like radiolucent defects .
Carcinogenicity
Iodoform has been included in the NCI daily bioassay program. According to their summary: the high and low time-weighted average daily dosages of iodoform were, respectively, 142 and 71 mg/kg for male rats, 55 and 27 mg/kg for female rats, and 93 and 47 mg/kg for male and female mice. A significant positive association between dosage and mortality was observed in male rats but not in female rats or in mice of either sex. Adequate numbers of animals in all groups survived sufficiently long to be at risk from late-developing tumors. No statistical significance could be attributed to the incidences of any neoplasms in rats or mice of either sex when compared to their respective controls. Under the conditions of this bioassay, no convincing evidence was provided for the carcinogenicity of iodoform in Osborne–Mendel rats or B6C3F1 mice.
Metabolism
It is expected to be oxidized to iodine [L2646].
Purification Methods
Crystallise it from MeOH, EtOH or EtOH/EtOAc. It is steam volatile. It is a disinfectant. [Beilstein 1 IV 97.]
Properties of Iodoform
| Melting point: | 119 °C |
| Boiling point: | 218°C |
| Density | 4.008 g/mL at 25 °C(lit.) |
| refractive index | 1.4975 (estimate) |
| storage temp. | -20°C |
| solubility | chloroform: soluble5% |
| form | Crystalline Powder |
| color | Bright yellow to dark yellow |
| Odor | char. pungent odor |
| Water Solubility | Insoluble. <0.1 g/100 mL at 24 ºC |
| Sensitive | Light Sensitive |
| Merck | 14,5033 |
| BRN | 1697010 |
| Exposure limits | ACGIH: TWA 0.6 ppm NIOSH: TWA 0.6 ppm(10 mg/m3) |
| Stability: | Stable. Incompatible with strong oxidizing agents, reducing agents. May explode when heated. |
| CAS DataBase Reference | 75-47-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, triiodo-(75-47-8) |
| EPA Substance Registry System | Iodoform (75-47-8) |
Safety information for Iodoform
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Iodoform
Iodoform manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Iodoform 99% CAS 75-47-8View Details
Iodoform 99% CAS 75-47-8View Details
75-47-8 -
 IODOFORMView Details
IODOFORMView Details
75-47-8 -
 IodoformView Details
IodoformView Details
75-47-8 -
 IodoformView Details
IodoformView Details
75-47-8 -
 IodoformView Details
IodoformView Details
75-47-8 -
 IodoformView Details
IodoformView Details
75-47-8 -
 IODOFORM 99 %View Details
IODOFORM 99 %View Details
75-47-8 -
 Iodoform ( 75-47-8 )View Details
Iodoform ( 75-47-8 )View Details
100-51-6
