Indoline
Synonym(s):1-Azaindan;2,3-Dihydroindole
- CAS NO.:496-15-1
- Empirical Formula: C8H9N
- Molecular Weight: 119.16
- MDL number: MFCD00005705
- EINECS: 207-816-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-27 13:07:25
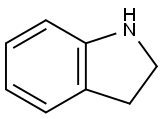
What is Indoline?
Chemical properties
2,3-Dihydroindole [496-15-1], indoline C8H9N, Mr 119.16, bp 229 – 230 ℃(101.3 kPa), is a colorless liquid, which is volatile in steam and soluble in diethyl ether, acetone, and benzene, but only slightly soluble in water. Indoline is obtained by hydrogenation of indole or by catalytic cyclodehydration of 2-(2-aminophenyl)ethanol. A range of pharmaceuticals, as well as fungicides and bactericides, can be produced from indoline.
The Uses of Indoline
As an indole derivative, Indoline can be used in the preparation of various medicinal compounds such as potential α1-adrenoceptor (α1-AR) antagonists.
The Uses of Indoline
Indoline is used in perfumery and in preparing tryptophan, one of the 20 amino acids commonly found in animal proteins. It has important application in the industry of plant growth. It is used to prepare indoleacetic acid (auxin) and other plant growth substances which help the development of roots in plant. Indole and its derivatives are widely used in making perfumes, dyes, agrochemicals and medicines.
The Uses of Indoline
Reactant for preparation of:
- Inhibitors of NOD1-Induced Nuclear Factor-κB Activation
- Sphingosine-1-phosphate 4(S1P4) receptor antagonists
- Cytotoxic cell cycle inhibitors
- 2-Aminopyridines
- PET agent for imaging of protein kinase C (PKC)
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- α4β2-Nicotinic acetylcholine receptor-selective partial agonists
- mGlu4 positive allosteric modulators
- Bacterial biofilm inhibitors
- Serotonin 5-HT6 receptor antagonists
Definition
ChEBI: Indoline is a member of indoles.
Synthesis Reference(s)
Journal of the American Chemical Society, 96, p. 7812, 1974 DOI: 10.1021/ja00832a035
Synthetic Communications, 13, p. 489, 1983 DOI: 10.1080/00397918308081827
Synthesis
Indoline can be produced from the reaction of indole,zinc and 85% phosphoric acid.
Properties of Indoline
| Melting point: | -21 °C |
| Boiling point: | 220-221 °C (lit.) |
| Density | 1.063 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 199 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 5g/l |
| form | liquid (clear) |
| pka | 5.20±0.20(Predicted) |
| color | clear dark brown |
| Odor | a mild, delicate, but extremely tenacious floral odor |
| Water Solubility | 5 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| BRN | 111915 |
| CAS DataBase Reference | 496-15-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Indole, 2,3-dihydro-(496-15-1) |
| EPA Substance Registry System | 1H-Indole, 2,3-dihydro- (496-15-1) |
Safety information for Indoline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Indoline
Indoline manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 496-15-1 INDOLINE 99%View Details
496-15-1 INDOLINE 99%View Details
496-15-1 -
 Indoline 99%View Details
Indoline 99%View Details
496-15-1 -
 496-15-1 99%View Details
496-15-1 99%View Details
496-15-1 -
 Indoline 98% (GC) CAS 496-15-1View Details
Indoline 98% (GC) CAS 496-15-1View Details
496-15-1 -
 Indoline CAS 496-15-1View Details
Indoline CAS 496-15-1View Details
496-15-1 -
 Indoline, 99% CAS 496-15-1View Details
Indoline, 99% CAS 496-15-1View Details
496-15-1 -
 Indoline CAS 496-15-1View Details
Indoline CAS 496-15-1View Details
496-15-1 -
 Indoline (CAS No.496-15-1), Purity: 99%, Technical GradeView Details
Indoline (CAS No.496-15-1), Purity: 99%, Technical GradeView Details
496-15-1
