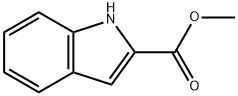
Ethyl indole-2-carboxylate
Synonym(s):2-Ethoxycarbonylindole;NSC 10076
- CAS NO.:3770-50-1
- Empirical Formula: C11H11NO2
- Molecular Weight: 189.21
- MDL number: MFCD00005609
- EINECS: 223-206-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-11 15:28:25
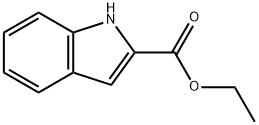
What is Ethyl indole-2-carboxylate?
Chemical properties
white to yellow crystals or crystalline powder
The Uses of Ethyl indole-2-carboxylate
Reactant for preparation of:• ;CRTH2 receptor antagonists1• ;Indoleamine 2,3-dioxygenase (IDO) inhibitors2• ;Cannabinoid CB1 receptor antagonists3• ;Inhibitors of Human Reticulocyte 15-Lipoxygenase-14• ;N-(benzoylphenyl)-1H-indole-2-carboxamides as potent antihypertriglyceridemic agents5• ;A antiproliferative agent against human leukemia K562 cells6• ;Inhibitors of p38 MAP kinase7• ;Acetolactate synthase inhibitors8
The Uses of Ethyl indole-2-carboxylate
Reactant for preparation of:CRTH2 receptor antagonists;Indoleamine 2,3-dioxygenase (IDO) inhibitors;Cannabinoid CB1 receptor antagonists; Inhibitors of Human Reticulocyte 15-Lipoxygenase-1;N-(benzoylphenyl)-1H-indole-2-carboxamides as potent antihypertriglyceridemic agents;A antiproliferative agent against human leukemia K562 cells;Inhibitors of p38 MAP kinase; Acetolactate synthase inhibitors.
The Uses of Ethyl indole-2-carboxylate
2-Ethoxycarbonylindole is a building block that has been used as a reactant for the preparation of pyridazinoindole derivatives that have antimicrobial activities.
Preparation
Indole-2-carb-oxy-lic acid (0.50 g, 3.1 mmol) was dissolved in SOCl2 (19 ml) at 0°C. After stirring for 1 h, the solution was rotary evaporated, and the resulting oil was added to absolute ethanol (17 ml) at room temperature. After stirring overnight, the solution was vacuum filtered to yield ethyl 1H-indole-2-carboxyl-ate as a beige solid, which was recrystallized from methanol to yield 0.54 g (2.9 mmol, 93%) of the product[1].
References
[1] Will E. Lynch , Clifford W. Padgett, Christine R. Whitlock . “Ethyl 1H-indole-2-carboxyl-ate.” IUCrData 5 (2020): Article x201205.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 39, p. 1152, 1991 DOI: 10.1248/cpb.39.1152
structure and hydrogen bonding
Lynch et al. report the crystal structure of ethyl 1H-indole-2-carboxyl-ate, which forms a hydrogen-bonded dimer. The hydrogen bonding occurs between N atoms of the indole ring and the keto oxygen atoms with an R(10) synthon. The hydrogen bond between N1 and O2i is characterized by an N…O separation of 2.877 Å;, and the ring motifs are placed on inversion centres in the space group P21/c. The crystal structure exhibits a classic herringbone pattern, with the blocks of hydrogen-bonded dimers and the zigzag running along the b-axis direction. The mol-ecule is nearly planar, with a r.m.s.d. of 0.028 Å for the non-hydrogen atoms. No other short contacts or π–π inter-actions are observed in the crystal[1].
Properties of Ethyl indole-2-carboxylate
| Melting point: | 122-125 °C (lit.) |
| Boiling point: | 324.47°C (rough estimate) |
| Density | 1.1596 (rough estimate) |
| refractive index | 1.5012 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | Crystals or Crystalline Powder |
| pka | 15.01±0.30(Predicted) |
| color | White to yellow |
| BRN | 146395 |
| InChI | InChI=1S/C11H11NO2/c1-2-14-11(13)10-7-8-5-3-4-6-9(8)12-10/h3-7,12H,2H2,1H3 |
| CAS DataBase Reference | 3770-50-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-indole-2-carboxylic acid, ethyl ester(3770-50-1) |
Safety information for Ethyl indole-2-carboxylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl indole-2-carboxylate
| InChIKey | QQXQAEWRSVZPJM-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC=C2)C=C1C(OCC)=O |
Ethyl indole-2-carboxylate manufacturer
Anand Agencies
ASM Organics
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 3770-50-1 Ethyl-Indole-2-Carboxylate 99%View Details
3770-50-1 Ethyl-Indole-2-Carboxylate 99%View Details
3770-50-1 -
 3770-50-1 99%View Details
3770-50-1 99%View Details
3770-50-1 -
 Ethyl Indole-2-carboxylate CAS 3770-50-1View Details
Ethyl Indole-2-carboxylate CAS 3770-50-1View Details
3770-50-1 -
 Ethyl indole-2-carboxylate CAS 3770-50-1View Details
Ethyl indole-2-carboxylate CAS 3770-50-1View Details
3770-50-1 -
 Ethyl indole-2-carboxylate 98% CAS 3770-50-1View Details
Ethyl indole-2-carboxylate 98% CAS 3770-50-1View Details
3770-50-1 -
 Ethyl indole-2-carboxylate CAS 3770-50-1View Details
Ethyl indole-2-carboxylate CAS 3770-50-1View Details
3770-50-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1