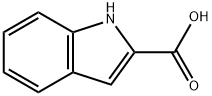
Indole-4-carboxaldehyde
Synonym(s):4-Formylindole;NSC 337264
- CAS NO.:1074-86-8
- Empirical Formula: C9H7NO
- Molecular Weight: 145.16
- MDL number: MFCD01632221
- EINECS: 625-162-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
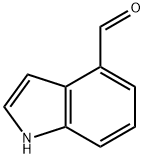
What is Indole-4-carboxaldehyde?
Chemical properties
White to dark brown solid
The Uses of Indole-4-carboxaldehyde
Indole-4-carboxaldehyde is usually used as reactant in Biginelli reaction,preparation of antitumor agents,intramolecular Friedel-Crafts acylation,preparation of inhibitors of cell division in E. Coli,synthesis of Hantzsch pyridine-containing Schiff bases.
The Uses of Indole-4-carboxaldehyde
4-Indolecarbaldehyde is a synthetic intermediate used for pharmaceutical synthesis. Also used as reactant in Biginelli reaction, synthesis of aurora kinase A inhibitors, preparation of antitumor agents, intermolecular Friedel-Crafts acylation, preparation of inhibitors of cell division in E. coli and synthesis of Hantzsch pyridine-containing Schiff bases.
What are the applications of Application
4-formyl Indole is a synthetic intermediate
Definition
ChEBI: Indole-4-carbaldehyde is a heteroarenecarbaldehyde that is indole in which the hydrogen at position 4 has been replaced by a formyl group. It has a role as an algal metabolite. It is a member of indoles and a heteroarenecarbaldehyde.
Synthesis Reference(s)
The Journal of Organic Chemistry, 46, p. 1752, 1981 DOI: 10.1021/jo00321a053
Tetrahedron, 39, p. 3695, 1983 DOI: 10.1016/S0040-4020(01)88608-7
General Description
Indole-4-carboxaldehyde participates in the synthesis of arcyriacyanin A. Synthesis of 4-indole-4-carboxaldehyde has been reported. Intramolecular Friedel-Crafts (FC) acylation of indole-4-carboxaldehyde has been reported.
Synthesis
Tetrapropylammonium perruthenate (0.3 g, 0.85 mmol) was added in portions to a mixture of alcohol product from 1H-Indole-4-Methanol (2.5 g, 17 mmol), N-methylmorpholine N-oxide (3.0 g, 25 mmol) and 4 A molecular sieves (3.0 g) in anhydrous methylene chloride (30 mL) at room temperature. The mixture was stirred at room temperature under nitrogen for 1 h and then filtered. The filtrate was concentrated in vacuo, and the residue was purified by chromatography (SiO2, CH2Cl2) to provide Indole-4-carboxaldehyde as a white powder (2.0 g, 80percent): 1H NMR (300 MHz, CDCl3) δ10.2 (s, 1H), 8.52 (br s, 1H), 7.64-7.69 (m, 2H), 7.31-7.44 (m, 3H), CI MS m/z=146 [C9H7NO+H]+.
Properties of Indole-4-carboxaldehyde
| Melting point: | 139-143 °C(lit.) |
| Boiling point: | 339.1±15.0 °C(Predicted) |
| Density | 1.278±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMF: 10 mg/ml; DMSO: 3 mg/ml; Ethanol: 10 mg/ml; Ethanol:PBS(pH 7.2) (1:1): 0.5 mg/ml |
| pka | 15.84±0.30(Predicted) |
| form | powder to crystal |
| color | Light yellow to Brown |
| Water Solubility | Soluble in ethanol and acetone. Insoluble in water. |
| Sensitive | Air Sensitive |
| InChI | InChI=1S/C9H7NO/c11-6-7-2-1-3-9-8(7)4-5-10-9/h1-6,10H |
| CAS DataBase Reference | 1074-86-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-indole-4-carboxaldehyde(1074-86-8) |
Safety information for Indole-4-carboxaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Indole-4-carboxaldehyde
| InChIKey | JFDDFGLNZWNJTK-UHFFFAOYSA-N |
| SMILES | N1C2=C(C(C=O)=CC=C2)C=C1 |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Indole-4-carboxaldehyde CAS 1074-86-8View Details
Indole-4-carboxaldehyde CAS 1074-86-8View Details
1074-86-8 -
 Indole-4-carboxaldehyde 98% (HPLC) CAS 1074-86-8View Details
Indole-4-carboxaldehyde 98% (HPLC) CAS 1074-86-8View Details
1074-86-8 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
