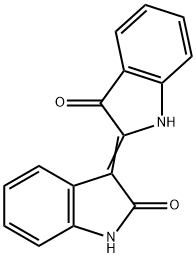
INDIRUBIN-3'-MONOXIME
Synonym(s):Indirubin-3ʹ-monoxime - CAS 160807-49-8 - Calbiochem;Indirubin-3′-monoxime
- CAS NO.:160807-49-8
- Empirical Formula: C16H11N3O2
- Molecular Weight: 277.28
- MDL number: MFCD02683594
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
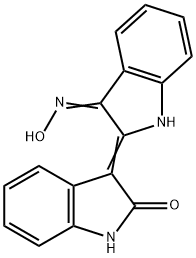
What is INDIRUBIN-3'-MONOXIME?
Description
Indirubin-
Chemical properties
Dark Red Solid
The Uses of INDIRUBIN-3'-MONOXIME
A potent inhibitor of GSK-3? (IC50=22nM). Also inhibits CDK1 (IC50=180nM) and CDK (IC50=100nM). It reversibly arrests asynchronous HBL-100 cells at G2. It induces apoptosis in the mammary carcinoma cell line MCF-7 (10üM).
The Uses of INDIRUBIN-3'-MONOXIME
Indirubin-3′-oxime has been used in the inhibition of glycogen synthase kinase 3 in human monocytic cell line, THP-1.
What are the applications of Application
Indirubin-3′-monoxime is A potent inhibitor of GSK-3β, Cdk1, and Cdk5
Definition
ChEBI: Indirubin-3'-monoxime is a member of the class of biindoles that is indirubin in which the keto group at position 3' has undergone condensation with hydroxylamine to form the corresponding oxime. It has a role as an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor, an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor, an osteogenesis regulator, a neuroprotective agent and an anti-obesity agent. It is a member of oxindoles, a bisindole, a ring assembly, a ketoxime and an alkaloid.
Biological Activity
Protein kinase inhibitor: inhibits cyclin-dependent kinases (IC 50 = 0.18-3.33 μ M) and GSK-3 β (IC 50 = 0.19 μ M). Inhibits CDK5- and GSK-3 β -mediated tau phosphorylation, a process over-active in Alzheimer disease states. Also inhibits AMPK, LCK and SGK. Induces cell cycle arrest and inhibits cell proliferation.
Biochem/physiol Actions
Indirubin-3′-oxime is a cyclin-dependent kinase inhibitor which functions by competing with ATP for binding to the catalytic subunit; exhibits antiproliferative activity leading to G2/M arrest in many cell lines and G1/S arrest in Jurkat cells.
storage
Store at RT
References
1) Leclerc?et al. (2001),?Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors?; J. Biol. Chem.,?276?251 2) Damiens?et al. (2001),?Anti-mitotic properties of indirubin-3′-monoximine, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest; Oncogene,?20?3786 3) Kim?et al. (2011),?Indirubin-3′-monoxime, a derivative of a chinese antileukemia medicine, inhibits angiogenesis; J. Cell. Biochem.,?112?1384 4) Ding?et al. (2010),?Indirubin-3′-monoxime rescues spatial memory deficits and attenuates beta-amyloid-associated neuropathology in a mouse model of Alzheimer’s disease; Neurobiol. Dis.,?39?156
Properties of INDIRUBIN-3'-MONOXIME
| Melting point: | 247-249°C |
| Boiling point: | 532.2±50.0 °C(Predicted) |
| Density | 1.50 |
| storage temp. | room temp |
| solubility | DMSO: >10 mg/mL |
| form | solid |
| pka | 8.66±0.20(Predicted) |
| color | Dark red or brown |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 160807-49-8 |
Safety information for INDIRUBIN-3'-MONOXIME
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for INDIRUBIN-3'-MONOXIME
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Indirubin-3′-monoxime CAS 160807-49-8View Details
Indirubin-3′-monoxime CAS 160807-49-8View Details
160807-49-8 -
 Indirubin-3′-oxime CAS 160807-49-8View Details
Indirubin-3′-oxime CAS 160807-49-8View Details
160807-49-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1