Indirubin
Synonym(s):Coroupitine B;Indigo Red;Indigopurpurin
- CAS NO.:479-41-4
- Empirical Formula: C16H10N2O2
- Molecular Weight: 262.26
- MDL number: MFCD00221745
- EINECS: 610-392-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
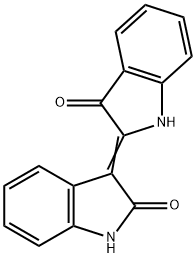
What is Indirubin?
Description
A further alkaloid isolated from the matured fruit of Couroupita guianensis, this base forms red crystals from EtOH and melts above 340°C. It forms the Nacetyl derivative, m.p. 186°C. The full structure has not yet been established.
Description
Indirubin, an indigo dye, is a biomolecule found in the African and Asian shrub Indigofera arrecta. It is variously known as indigo red; indigo naturalis; qing dai; or Natal, Bengal, or Java indigo. It is also bacterially produced in the urine of humans and other mammals.
I. arrecta has been used as a folk medicine in Africa and Asia for centuries. In particular, Congolese healers use it in combination with other herbs to treat epilepsy. Indirubin itself shows biological activity and has been studied as a treatment for diseases ranging from cancers to colitis.
Recently, a consortium of researchers in Belgium, Tanzania, and the Democratic Republic of the Congo, led by Najat Aourz at Vrije Universiteit Brussel, became interested in how the Congolese herb cocktail controls epilepsy. Using zebrafish larvae as an epilepsy model, they deduced that indirubin is the ingredient that protects the larvae against chemically induced seizures. Indirubin similarly prevents induced seizures in rats and mice.
The researchers then sought to identify the protein that indirubin blocks to inhibit seizures. The culprit turned out to be glycogen synthase kinase-3, an enzyme that the body uses to regulate energy storage. The exact mechanism by which indirubin disables the enzyme has not yet been established.
Physical properties
Appearance: dark red acicular crystal with sublimate, odorless, and tasteless. Solubility: slightly soluble in dimethyl sulfoxide (DMSO) or tetrahydrofuran, very slightly soluble in chloroform or acetone, and insoluble in ethanol, ethyl ether, or water. Melting point: 348–353 °C. The stability of indirubin is poor that it needs to be stored away from light and thermal environment.
History
The report in 1951 showed that indirubin can inhibit eosinophils in the blood of guinea pigs. But this report did not attract attention. Indirubin is the effective component of the traditional Chinese medicine Angelica aloe pill. Since 1966, the scientists in the Institute of Hematonosis, Chinese Academy of Medical Sciences Research, started the research of therapying chronic myelocytic leukemia with the TCM rules using Angelica aloe pills, which had certain effect. Angelica aloe pill consisted of 11 herbs, such as Angelica, Aloe, Rhizoma Coptidis, and natural indigo. It was identified that natural indigo was the effective component of Angelica aloe pill. However, the active ingredient of natural indigo was indirubin.
Indirubin is a novel antileukemia drug, which was found by the medical scientists in China in the middle of the 1970s. It has the effects of antibacterial, antiinflammatory, antitumor, and enhancing immune functions. The compound has
been applied to the clinical treatment of chronic myeloid leukemia. Indirubin has
the characteristics of reliable clinical curative effect, small side effects, and no obvious inhibitory effect on the bone marrow.
The Uses of Indirubin
Indigopurpurin is a purple 3,2-bisindole derivative and was shown to exhibit inhibitory allergic contact dermatitis via regulating T helper (Th)-mediated immune system in DNCB-induced model.
The Uses of Indirubin
An inhibitor of GSK-3β and cyclin-dependent kinases.
The Uses of Indirubin
Indigopurpurin is a purple 3,2-bisindole derivative and was shown to exhibit inhibitory allergic contact dermatitis via regulating T helper (Th)-mediated immune system in DNCB-induced model. A possible glycogen synthase kinase-3 (GSK-3) inhibitor the active component of a traditional Congolese antiepilepsy treatment.
What are the applications of Application
Indirubin is an inhibitor of GSK-3β and cyclin-dependent kinases
Indications
Indirubin is contained in the fourth volume of the book, “National standards for chemical drugs.”
Indirubin tablets are used for the therapy of chronic myelocytic leukemia in clinical.
General Description
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG
Pharmacology
The effect of indirubin has anti-inflammatory, antibacterial, detoxification, enhancing immune function anticancer. Indirubin has moderate inhibitory effect for animal-transplanted tumor. 200? mg/kg indirubin subcutaneous or intraperitoneal injection, once daily for 6 consecutive days, had the inhibition effect to rat W256 tumor cancer sarcoma; the inhibition rates were 47–52% and 50–58%, respectively. The chemotherapy index of intraperitoneal injection was 2.23. However, the inhibition rate of 500?mg/kg indirubin by gavage was only 23–33%. Indirubin by perfusion can prolong the survival time of W526 rat. The inhibition rate of Lewis mice’s lung cancer was 43% for 500?mg/kg indirubin orally, once a day for 9–10?days. Indirubin has some inhibition effects on mice breast cancer, but no obvious effect for L1212, P388, and L1210 of lymphocytic leukemia in mice.
The effect of indirubin on chronic myelogenous leukemia is very obvious,
which is similar to first clinical choice of Maryland. Indirubin has the advantages of
fast curative effect, no obvious inhibition effect of the bone, small bone marrow toxicity, and low side effects. Indirubin may have the effect of improving adrenocorticotropic hormone. In pathological conditions, such as inflammatory diarrhea, protein
metabolism disorder, kidney disease, leukemia, and other tumors, urinary excretion of
indirubin increased. Indirubin can inhibit synthesis of DNA and destroy the leukemia
cells. It was found by electron microscopy that juvenile cells reduced, even disappear
completely, with indirubin administration. Indirubin can significantly reduce the size
of spleen, increase the concentration of hemoglobin to normal level, and reduce the
swelling liver. In addition, indirubin can also enhance the phagocytic ability of animal
mononuclear macrophages, which play a role in body immune reaction. So the anticancer effect of this product may be related to improving the body’s immunity.
Clinical Use
Indirubin is mainly used for chronic myeloid leukemia; its total effective rate was 87.3%. The effect of indirubin to decrease white blood cells is similar to Maryland. The effect of indirubin to reduce liver is better than that of Maryland. But the remission role of the blood and bone marrow is worse than Maryland, and no cross resistance with Maryland. Indirubin could be used for abnormal bone marrow hyperplasia and eosinophilia.
in vitro
indirubin exerted its inhibitory effects not only on interferon-γ production by human myelomonocytic hbl-38 cells but also on interferon-γ and interleukin-σ production by murine splenocytes with no influence on the proliferation of either cells [1].
in vivo
because of indirubin’s inhibitory activity on interferon-γ production, the authors further investigated the effects of indirubin on 2,4,6-trinitro-l-chlorobenzene-induced delayed-type hypersensitivity. when injected intraperitoneally, indirubin significantly inhibited the ear swelling of tncb-elicited mice. moreover,the amount of interferon-γ in the culture supernatants of elicited mouse lymphocytes was inhibited by indirubin treatment [1].
References
Sen, Mahato, Dutta, Tetrahedron Lett., 609 (1974)
Properties of Indirubin
| Melting point: | 350°C(lit.) |
| Boiling point: | 496.6±45.0 °C(Predicted) |
| Density | 1.417±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Heated) |
| form | Purple powder. |
| appearance | red to deep purple crystals or powder |
| pka | 9.13±0.20(Predicted) |
| color | Very Dark Red |
| λmax | 540nm(DMSO)(lit.) |
| InChI | InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,17H,(H,18,20) |
| CAS DataBase Reference | 479-41-4(CAS DataBase Reference) |
Safety information for Indirubin
Computed Descriptors for Indirubin
| InChIKey | CRDNMYFJWFXOCH-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC=C2)C(=C2C(=O)C3=C(N2)C=CC=C3)C1=O |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Indirubin 97% (HPLC) CAS 479-41-4View Details
Indirubin 97% (HPLC) CAS 479-41-4View Details
479-41-4 -
 Indirubin CAS 479-41-4View Details
Indirubin CAS 479-41-4View Details
479-41-4 -
 Indirubin CAS 479-41-4View Details
Indirubin CAS 479-41-4View Details
479-41-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
