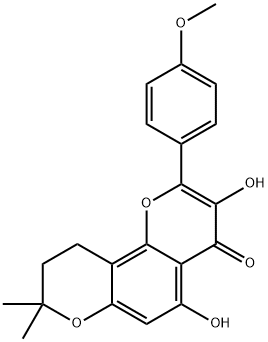
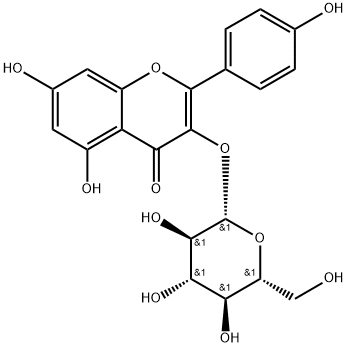
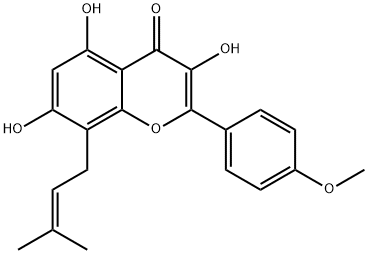
Icaritin
Synonym(s):3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one
- CAS NO.:118525-40-9
- Empirical Formula: C21H20O6
- Molecular Weight: 368.38
- MDL number: MFCD22422519
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Icaritin?
Chemical properties
Yellow crystalline powder, soluble in methanol, ethanol, DMSO and other organic solvents, derived from epimedium.
The Uses of Icaritin
Icaritin is a hydrolytic product of Icariin, a traditional Chinese herbal medicine extracted from the Epimedium genus. Icaritin and Desmethylicaritin, is two metabolites of Icariin, dramatically inhibit the growth of most malignant cells. They also have significant antiangiogenesis properties, inhibiting or eliminating entirely the development of new malignant cells. Icaritin has been used in trials studying the treatment of Solid Tumors, Metastatic Breast Cancer, and Hepatocellular Carcinoma (HCC).
Biochem/physiol Actions
Icaritin is a component of Epimedium flavonoid isolated from Herba Epimedii, which enhances osteoblastic differentiation of mesenchymal stem cells (MSCs) while it inhibits adipogenic differentiation of MSCs by inhibiting PPAR-g pathway. Icaritin has no effect on MSCs proliferation. Also, icaritin potently inhibits chronic myeloid leukemia (CML) and breast cancer cells proliferation most likely by modulation of MAPK/ERK/JNK and JAK2/STAT3 /AKT signaling. As other flavonoids, icaritin may exert estrogen-like activities.
Synthesis
The novel total synthesis of icaritin, naturally occurring with important bioactive 8-prenylflavonoid, was performed via a reaction sequence of 8 steps including Baker-Venkataraman reaction, chemoselective benzyl or methoxymethyl protection, dimethyldioxirane (DMDO) oxidation, O-prenylation, Claisen rearrangement and deprotection, starting from 2,4,6-trihydroxyacetophenone and 4-hydroxybenzoic acid in overall yields of 23%. The key step was Claisen rearrangement under microwave irradiation.
Total Synthesis of Icaritin via Microwave-assistance Claisen Rearrangement
Mode of action
Icaritin is a flavonoid first isolated from the Chinese herb H. epimedii that demonstrates anticancer activity against a variety of tumor cell lines. Icaritin was shown in vitro to sustain extracellular signal–regulated kinase (ERK) activity through stimulating estrogen receptors. Prolonged ERK activation arrested the cell cycle in the G2 stage and subsequently triggered apoptosis. It has been shown to inhibit fatty acid synthase, reducing IGF-1-induced activation of STAT3 in several melanoma cell lines.
Properties of Icaritin
| Melting point: | 239℃ |
| Boiling point: | 582.0±50.0 °C(Predicted) |
| Density | 1.359 |
| Flash point: | 206.7℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| pka | 6.44±0.40(Predicted) |
| color | light yellow to dark yellow |
| λmax | 368nm(MeOH)(lit.) |
| InChI | InChI=1S/C21H20O6/c1-11(2)4-9-14-15(22)10-16(23)17-18(24)19(25)20(27-21(14)17)12-5-7-13(26-3)8-6-12/h4-8,10,22-23,25H,9H2,1-3H3 |
Safety information for Icaritin
Computed Descriptors for Icaritin
| InChIKey | TUUXBSASAQJECY-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(OC)C=C2)OC2=C(C/C=C(/C)\C)C(O)=CC(O)=C2C(=O)C=1O |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Icaritin 98% (HPLC) CAS 118525-40-9View Details
Icaritin 98% (HPLC) CAS 118525-40-9View Details
118525-40-9 -
 Icaritin CAS 118525-40-9View Details
Icaritin CAS 118525-40-9View Details
118525-40-9 -
 Icaritin CAS 118525-40-9View Details
Icaritin CAS 118525-40-9View Details
118525-40-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1