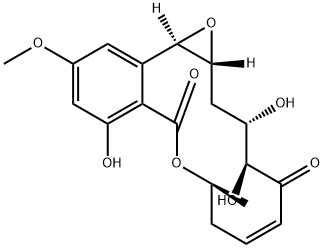
HYPOTHEMYCIN
Synonym(s):3H-Oxireno[k][2]benzoxacyclotetradecin-5,11(2H,4H)-dione,1a,8,9,15b-tetrahydro-3,4,12-trihydroxy-14-methoxy-9-methyl-,(1aR,3S,4S,6Z,9S,15bR)-;Hypothemycin - CAS 76958-67-3 - Calbiochem;NSC 354462;NSC354462
- CAS NO.:76958-67-3
- Empirical Formula: C19H22O8
- Molecular Weight: 378.37
- MDL number: MFCD08457932
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14
What is HYPOTHEMYCIN?
Description
Hypothemycin (76958-67-3) is a potent and selective inhibitor of MEK.1-3?Acts via covalent binding to Cys residue.2?In cells, hypothemycin inhibits the MEK-ERK axis with sufficient selectivity to normalize transformed phenotypes3. Inhibits TNFα production in LPS-stimulated macrophages.4
The Uses of HYPOTHEMYCIN
Hypothemycin is a potent and selective inhibitor of threonine/tyrosine-specific kinase, MEK, and other protein kinases that contain a conserved cysteine residue in the ATP-binding site in in vitro studies.
What are the applications of Application
Hypothemycin is an antifungal selective inhibitor of MEK
General Description
A cell-permeable cis-enone resorlic acid lactone (RAL) and (5Z)-7-Oxozeaenol (Cat. No. 499610) analog that inhibits several kinases via an initial affinity binding to the ATP pocket, followed by a covalent adduct formation with a conserved ATP site cysteine residue of the targeted enzymes, including, but not limited to, Flt1/VEGFR-1, Flt3, cKit(D816V), MAPK1, MAPK2, MEK1, MKK6, PKCμ, PKD2, PRAK, and Tak1 (≥90% inhibition at 2 μM). Hypothemycin is also reported to inhibit two non-RAL targets (99% and 82% inhibition of TrkA and TrkB, respectively, at 2 μM), while one of the 46 putative cis-enone RALs targets, namely GSK-3α, is shown not to be affected. Hypothemycin inhibits multiple kinases involved in MAP kinase pathways and is therefore particularly effective in inhibiting BRAF(V600E)-dependent tumor growth. (5Z)-Zeaenol (Cat. No. 499609) may serve as a negative control.
Biochem/physiol Actions
Hypothemycin, one of the highly oxygenated analogues in the group of 14-membered resorcylic acid lactones (RAL), has minor antifungal and cytotoxic activity and exhibits an in vitro anti-malarial activity with an IC50 of 2.2 μg/mL. Hypothemycin is also reported to selectively and irreversibly inhibit protein kinases that contain a conserved cysteine residue (Cys166) that is located within the ATP-binding domain. Though this group accounts for less then 10% of all identified kinases, there are several targets implicated in aberrant cellular proliferation such as ERKs, MEK, FMS-like tyrosine kinase protein (FLT), and platelet-derived growth factor receptors (PDGFR). In cell culture, hypothemycin displays potent cytotoxicity against cancer cell lines that are dependent on certain activating kinase mutations. Additionally, hypothemycin demonstrates significant tumor growth inhibition in at least three separate murine xenograft models. Hypothemycin also inhibits the production of several cytokines such as IL2, IL6, IFNγ, and TNFα.
References
1) Zhou et al. (1999), Resorcylic acid lactones: naturally occurring potent and selective inhibitors of MEK; J. Antibiot., 52 1086 2) Schirmer et al. (2006), Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides; Proc. Natl. Acad. Sci. USA, 103 4234 3) Fukazawa et al. (2010), The resorcylic acid lactone hypothemycin selectively inhibits the mitogen-activated protein kinase kinase-extracellular signal-regulated kinase pathway in cells; Biol. Pharm. Bull., 33 168 4) Park et al. (2015), Hypothemycin inhibits tumor necrosis factor-α production by tristetraprolin-dependent down-regulation of mRNA stability in lipopolysaccharide-stimulated macrophages; Int. Immunopharmacol., 29 863
Properties of HYPOTHEMYCIN
| Melting point: | 170-172℃ |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| form | Lyophilized solid |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for HYPOTHEMYCIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for HYPOTHEMYCIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Hypothemycin CAS 76958-67-3View Details
Hypothemycin CAS 76958-67-3View Details
76958-67-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
