hypochlorous acid
- CAS NO.:7790-92-3
- Empirical Formula: ClHO
- Molecular Weight: 52.46
- MDL number: MFCD00871734
- EINECS: 232-232-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:36
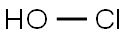
What is hypochlorous acid?
Description
Hypochlorous acid [7790-92-3], HOCl, Mr 52.5, is only moderately stable in aqueous solution. It is colorless when dilute and yellowish at higher concentrations. Hypochlorous acid is one of the most powerful oxidizing agents known. Hypochlorous acid solution decomposes exothermically. The main decomposition products are hydrochloric acid and oxygen.
Chemical properties
Greenish-yellow aqueous solution. Highly unstable, weak acid. Decomposes to hydrogen chloride and oxygen. Can exist only in dilute solutions.
Physical properties
Greenish-yellow aqueous solution; unstable; weak acid, pKa7.40 at 25°C;soluble in water.
The Uses of hypochlorous acid
Disinfectant.
Definition
ChEBI: Hypochlorous acid is a chlorine oxoacid with formula HOCl; a weak, unstable acid, it is the active form of chlorine in water. It has a role as a human metabolite, an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an EC 2.5.1.18 (glutathione transferase) inhibitor. It is a member of reactive oxygen species and a chlorine oxoacid. It is a conjugate acid of a hypochlorite.
Preparation
Hypochlorous acid is obtained by dissolving chlorine in water, or by addingbleaching powder or sodium hypochlorite to water. A better method of pro-duction is passing chlorine gas into a well-agitated suspension of mercuricoxide:
2Cl2 + 2HgO + H2O → HgO•HgCl2 + 2HOCl
or by distilling chlorine hexahydrate and mercuric oxide at low pressure:
2Cl2•6H2O + HgO → 2HOCl + HgCl2 + 5H2O
The latter process can yield a 25% acid solution.
Hypochlorous acid also may be obtained by hydrolysis of chlorine monoflu-oride:
ClF + H2O → H+ + F¯ + HOCl
The above reaction may be explosive and is not recommended for preparinghypochlorous acid.
Hazard
Irritant to skin and eyes.
Properties of hypochlorous acid
| Boiling point: | 125-130 °C(Press: 11 Torr) |
| Density | 1.1655 g/cm3 |
| solubility | soluble in H2O |
| form | stable only in solution |
| pka | pK (25°) 7.49 |
| color | green-yellow; stable
only in aqueous solution |
| EPA Substance Registry System | Hypochlorous acid (7790-92-3) |
Safety information for hypochlorous acid
Computed Descriptors for hypochlorous acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
