Lithium perchlorate
Synonym(s):Lithium cloricum;Perchloric acid, lithium salt
- CAS NO.:7791-03-9
- Empirical Formula: ClLiO4
- Molecular Weight: 106.39
- MDL number: MFCD00011079
- EINECS: 232-237-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
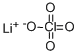
What is Lithium perchlorate?
Description
Lithium perchlorate,is an oxysalt that is a colorless, deliquescent crystal. Oxysalt “per-ate” compounds are loaded with excess oxygen and will readily give it up in a reaction. Lithium perchlorate is a powerful oxidizing agent. It has more available oxygen than does liquid oxygen on a volume basis. Lithium perchlorate has a specific gravity of 2.429, which is heavier than water, and is water soluble. It is a dangerous fire and explosion risk in contact with organic materials and is an irritant to skin and mucous membranes. The primary use of lithium perchlorate is as a solid rocket propellant. Chlorates are strong oxidizing agents. When heated, they give up oxygen readily. Contact with organic or other combustible materials may cause spontaneous combustion or explosion. They are incompatible with ammonium salts, acids, metal powders, sulfur, and finely divided organic or combustible substances.
Chemical properties
White crystalline powder
The Uses of Lithium perchlorate
The big advantage of lithium perchlorate is its high density and oxygen availability for combustion. A quite interesting methodology involves the use of lithium perchlorate anhydrides complexes in the acylation of activated aromatic compounds.Lithium perchlorate is frequently utilized as promoter to accelerate the acylation process and to increase the yield in the reaction catalyzed by metal triflates.However, lithium perchlorate itself can act as a very efficient catalyst in the acylation of variously substituted methoxy? and methylbenzenes with AAN affording the aryl ketones in 65%–99% yield. The exceptional activation is ascribed to the formation of a complex with a strong electrophilic character between lithium perchlorate and AAN in neat AAN. The observed para?regioselectivity can be interpreted in terms of the high steric requirement of the lithium perchlorate– AAN complex. A further important feature of this process is the possibility of quantitatively recovering and reusing the catalyst after activation. It must be underlined, however, that lithium perchlorate is an oxidizing compound and can undergo devastating explosions; consequently, it must be handled with maximum care.
The Uses of Lithium perchlorate
Used as an oxidizing agent.
The Uses of Lithium perchlorate
Oxidizing agent.
What are the applications of Application
Lithium perchlorate is an effective protein denaturant
General Description
Lithium perchlorate (LiClO4) is a colorless lithium salt. Upon crystallization from its aqueous solution, it affords lithium perchlorate trihydrate (LiClO4.3H2O). It can be synthesized by reacting lithium chloride with perchloric acid.
Flammability and Explosibility
Not classified
Battery Materials
Lithium perchlorate (LiClO4) is sufficiently soluble (beyond 1Min organic solvents, e.g., EC/DMC) and forms electrolyte solutions with good conductivity (about 9 mS·cm?1 in EC/DMC at ambient temperature). In organic solvents LiClO4 forms thicker solid electrolyte interface (SEI) layers than LiPF6 or LiBF4, but they are less resistive. This fact is attributed to the highly resistive LiF on the surface which is formed by hydrogen fluoride (HF) generated by hydrolysis of fluorine-containing anions, for example, LiBF4 and LiPF6, with traces of moisture and the existing SEI layer [62, 63]. Furthermore, it has a high anodic stability of up to 5.1 V on LiMn2O4 in EC/DMC and is less hygroscopic than LiPF6. Despite its many advantages, the high oxidation state of chlorine (VII) in ClO4 ? results in problems. LiClO4 solutions are thermally unstable and show explosion risks, especially in ethers.
Purification Methods
Crystallise it from water or 50% aqueous MeOH. It is rendered anhydrous by heating the trihydrate at 170-180o in an air oven. It can then be recrystallised twice from acetonitrile and again dried under vacuum [Mohammad & Kosower J Am Chem Soc 93 2713 1971]. SKIN IRRITANT.
Properties of Lithium perchlorate
| Melting point: | 236 °C (lit.) |
| Boiling point: | 430°C |
| Density | 1.13 g/mL at 20 °C |
| Flash point: | 400°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | powder |
| Specific Gravity | 2.43 |
| color | White |
| PH | 6.0-7.5 (25℃, 5%) |
| PH Range | 6.0 - 7.5 |
| Water Solubility | 600 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,5539 |
| Stability: | Strong oxidizer - contact with combustible material may cause fire. Incompatible with organic materials, combustible materials, strong reducing agents. |
| CAS DataBase Reference | 7791-03-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium perchlorate(7791-03-9) |
| EPA Substance Registry System | Perchloric acid, lithium salt (7791-03-9) |
Safety information for Lithium perchlorate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium perchlorate
Lithium perchlorate manufacturer
PARAD CORPORATION PRIVATE LIMITED
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

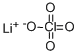


You may like
-
 Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
7791-03-9 -
 Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
7791-03-9 -
 Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
Lithium perchlorate, Anhydrous CAS 7791-03-9View Details
7791-03-9 -
 Lithium perchlorate 99% CAS 7791-03-9View Details
Lithium perchlorate 99% CAS 7791-03-9View Details
7791-03-9 -
 Lithium perchlorate CAS 7791-03-9View Details
Lithium perchlorate CAS 7791-03-9View Details
7791-03-9 -
 Lithium perchlorate CAS 7791-03-9View Details
Lithium perchlorate CAS 7791-03-9View Details
7791-03-9 -
 Crystals Laboratory Lithium Perchlorate, For Battery ManufacturingView Details
Crystals Laboratory Lithium Perchlorate, For Battery ManufacturingView Details
7791-03-9 -
 Lithium Perchlorate ARView Details
Lithium Perchlorate ARView Details
13453-78-6
