Hymexazol
Synonym(s):Hymexazol
- CAS NO.:10004-44-1
- Empirical Formula: C4H5NO2
- Molecular Weight: 99.09
- MDL number: MFCD00144468
- EINECS: 233-000-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
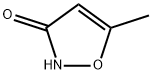
What is Hymexazol?
Chemical properties
White Solid
The Uses of Hymexazol
Hymexazol is used to control soil-borne diseases caused by Fusarium, Aphanomyces, Pythium, Corficium and Typhula spp. in rice, sugar beet, fodder beet, vegetables, cucurbits, ornamentals, carnations and forest tree seedlings. It is also used as a seed dressing and stimulates some plant growth.
The Uses of Hymexazol
Pesticide.
The Uses of Hymexazol
Agricultural fungicide and plant growth regulator.
Definition
ChEBI: A member of the class of isoxazoles carrying hydroxy and methyl substituents at positions 3 and 5 respectively. It is used worldwide as a systemic soil and seed fungicide for the control of diseases caused by Fusarium, Aphanomyces Pythium, and Corticium spp. in rice, sugarbeet, fodderbeet, vegetables, cucurbits, and ornamentals.
Synthesis Reference(s)
The Journal of Organic Chemistry, 48, p. 4307, 1983 DOI: 10.1021/jo00171a030
Metabolic pathway
Degradation of hymexazol in soil gave acetoacetamide and the product of rearrangement, 5-methyl-2(3H)-oxazolone.H owever, in plants the fungicide was principally converted into its O- and N-glucoside conjugates in the roots and shoots. The two main metabolites of hymexazol found in the urine of rats were the O-glucuronide and sulfate conjugates.
Degradation
Hymexazol is stable under alkaline conditions and relatively stable in acidic conditions. It is stable to sunlight and heat (PM). It should be noted that the parent molecule is tautomeric. Hymexazol is highly volatile and will be lost by volatilisation unless it is covered or incorporated into soil. The fungicide was completely biodegraded in natural water at 30 °C in 2 weeks and at 10-13 °C in 2 months (Rebenok and Kolesnikova, 1983). Hymexazol is stable in sunlight but it is readily degraded by ultraviolet light. Photolysis of an aqueous solution of the fungicide at 253.7 nm, using a low pressure Hg lamp, afforded the oxazolinone (2) as the major product and at least two unidentified minor components. The oxazolinone (2) has been found in soil studies as described below and is a product of rearrangement formed via an aziridinone intermediate as shown in Scheme 1 (Nakagawa et al., 1974).
Properties of Hymexazol
| Melting point: | 80°C |
| Boiling point: | 185.54°C (rough estimate) |
| Density | 1.2992 (rough estimate) |
| vapor pressure | 0.182 Pa (25 °C) |
| refractive index | 1.4170 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol, acetone, THF, chloroform |
| form | Powder |
| Water Solubility | 65,100 mg l-1 (20 °C) |
| pka | 5.91 (weak acid) |
| color | White to Orange |
| Sensitive | Light Sensitive |
| Merck | 14,4856 |
| CAS DataBase Reference | 10004-44-1(CAS DataBase Reference) |
| EPA Substance Registry System | Hymexazol (10004-44-1) |
Safety information for Hymexazol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Hymexazol
| InChIKey | KGVPNLBXJKTABS-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
10004-44-1 -
 Hymexazol 98% CAS 10004-44-1View Details
Hymexazol 98% CAS 10004-44-1View Details
10004-44-1 -
 Hymexazol 98% (HPLC) CAS 10004-44-1View Details
Hymexazol 98% (HPLC) CAS 10004-44-1View Details
10004-44-1 -
 3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
10004-44-1 -
 3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
3-Hydroxy-5-methylisoxazole CAS 10004-44-1View Details
10004-44-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1