116-09-6
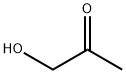
Product Name:
Hydroxyacetone
Formula:
C3H6O2
Synonyms:
1-Hydroxy-2-propanone;Acetol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless liquid with a peculiar odor; [Merck Index] Clear light yellow liquid; [Aldrich MSDS] |
|---|---|
| Boiling Point | 145.00 to 146.00 °C. @ 760.00 mm Hg |
| Melting Point | -17 °C |
| Solubility | 1.00E+06 mg/L @ 20 °C (exp) |
| Density | 1.079-1.085 (20°) |
| Vapor Pressure | 2.95 [mmHg] |
| Refractive Index | 1.419-1.430 |
| Kovats Retention Index | 681 625 652 652 655 632 631 668 668 620 625 640 |
| Chemical Classes | Other Classes -> Other Organic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
COMPUTED DESCRIPTORS
| Molecular Weight | 74.08 g/mol |
|---|---|
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 74.036779430 g/mol |
| Monoisotopic Mass | 74.036779430 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 40.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Hydroxyacetone is a propanone that is acetone in which one of the methyl hydrogens is replaced by a hydroxy group. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a member of propanones, a methyl ketone, a primary alcohol and a primary alpha-hydroxy ketone. It is functionally related to an acetone.