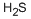CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs. Boiling point -60.2 °C. Shipped as a liquid confined under its own vapor pressure. Density (liquid) 8.3 lb / gal. Contact with the unconfined liquid can cause frostbite by evaporative cooling. Gas is very toxic by inhalation. Fatigues the sense of smell which cannot be counted on to warn of the continued presence of the gas. Prolonged exposure of closed containers to heat may result in their violent rupturing and rocketing. Rate of onset: Immediate & Delayed Persistence: Minutes to hours Odor threshold: 0.1 ppm Source/use/other hazard: Disinfectant lubricant/oils; interm for HC manufacture; deadens sense of smell. |
|---|---|
| Color/Form | Colorless gas [Shipped as a liquefied compressed gas]. |
| Odor | Strong odor of rotten eggs [Note: Sense of smell becomes rapidly fatigued & can NOT be relied upon to warn of the continuous presense of hydrogen sulfide]. |
| Taste | Sweetish taste |
| Boiling Point | -76.59 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -121.9 °F (EPA, 1998) |
| Flash Point | 500 °F |
| Solubility | 0.4 % (NIOSH, 2023) |
| Density | 0.916 at -76 °F (EPA, 1998) - Less dense than water; will float |
| Vapor Density | 1.19 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 15200 mmHg at 77.9 °F (EPA, 1998) |
| Henry's Law Constant | Henry's law constant = 0.0098 atm-cu m/mole at 25 °C |
| Stability/Shelf Life | Water solns of H2S are not stable, absorbed oxygen causes the formation of elemental sulfur, and the solns become turbid rapidly. |
| Autoignition Temperature | 500 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits highly toxic fumes of /oxides of sulfur/. |
| Viscosity | Gas at 101.325 KPa at 25 °C; 0.012 8 m Pa.S; 0.012 8 cP. |
| Corrosivity | Anhydrous hydrogen sulfide has low general corrosivity toward carbon steel, aluminum, Inconel, Stellite and 300-series stainless steels at moderate temperatures. Temperatures greater than ca 260 °C can produce severe sulfidation of carbon steel. Wet hydrogen sulfide can be quite corrosive to carbon steel. |
| Heat of Vaporization | Molar enthalpy of vaporization: 14.08 kJ/mol at 25 °C; 18.67 kJ/mol at -59.55 °C |
| pH | pH freshly prepared water soln: 4.5 |
| Ionization Potential | 10.46eV |
| Odor Threshold | Odor Threshold Low: 0.001 [ppm] Odor Threshold High: 0.13 [ppm] Detection odor threshold from AIHA (mean = 0.0094) |
| Refractive Index | INDEX OF REFRACTION (LIQ): 1.374 |
| Dissociation Constants | pKa1 = 7.04; pKa2 = 11.96 |
| Kovats Retention Index | 340 338 338 |
| Other Experimental Properties | Triple Point; 187.62 K; -85.5 °C; -122.0 °F: Absolute density, Gas at 101.325 kPa at 25 °C 1.506 kg/cu m: Relative density, Gas at 101.325 kPa at 25 °C (Air = 1) 1.188: Density, Liquid at Saturation Pressure at -60.3 °C 0.960 kg/l: Critical Temperature 373.56 K; 100.4 °C; 212.7 °F: Critical Volume 2.867 dm3/kg: Critical Density 0.349 kg/dm3: Critical Compressibility Factor 0.283: Latent Heat of Fusion at -85.5 °C 2 376.5 J/mol; 69 741.2 J/kg; 16.67 kcal/kg Thermal Conductivity, Gas at 101.325 kPa at 15.6 °C 0.014 004 W/(m.K); 33.5 x 10-6 cal/(sq cm °C) Solubility In Water at 101.325 kOPa at 25 °C 2.257 cu m/1 cu m water: Index of Refraction, Gas at 101.325 kPa, ND at 25 °C 1.000 584 5: Dielectric Constant; Gas at 0 °C, 101.325 kPa 1.004: Liquid at -78.5 °C 9.05. |
| Chemical Classes | Toxic Gases & Vapors -> Chemical Asphyxiants |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P284:Wear respiratory protection. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
COMPUTED DESCRIPTORS
| Molecular Weight | 34.08 g/mol |
|---|---|
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 33.98772124 g/mol |
| Monoisotopic Mass | 33.98772124 g/mol |
| Topological Polar Surface Area | 1 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Hydrogen sulfide (H2S) occurs naturally in crude petroleum, natural gas, volcanic gases, and hot springs. It can also result from bacterial breakdown of organic matter. It is also produced by human and animal wastes. Bacteria found in your mouth and gastrointestinal tract produce hydrogen sulfide from bacteria decomposing materials that contain vegetable or animal proteins. Hydrogen sulfide can also result from industrial activities, such as food processing, coke ovens, kraft paper mills, tanneries, and petroleum refineries. Hydrogen sulfide is a flammable, colorless gas with a characteristic odor of rotten eggs. It is commonly known as hydrosulfuric acid, sewer gas, and stink damp. People can smell it at low levels.