Hexetidine
Synonym(s):5-Amino-1,3-bis(2-ethylhexyl)hexahydro-5-methylpyrimidine;Hexetidine, mixture of stereoisomers;NSC 17764
- CAS NO.:141-94-6
- Empirical Formula: C21H45N3
- Molecular Weight: 339.6
- MDL number: MFCD00010428
- EINECS: 205-513-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
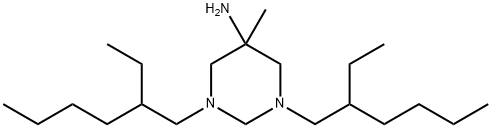
What is Hexetidine?
Chemical properties
Liquid.Soluble in methanol, benzene, and petroleum ether; insoluble in water.
Chemical properties
Hexetidine is a colorless or faint yellow-colored oily liquid with a characteristic amine odor.
Originator
Sterisil,Warner Lambert,US,1956
The Uses of Hexetidine
Hexetidine, mixture of stereoisomers is an antibacterial and antifungal agent. Used in plant-derived composite antimicrobial agent and application thereof in cosmetic.
The Uses of Hexetidine
Fungicide, bactericide, algicide, antistatic agent for synthetics, insect repellent, medicine (antifungal agent).
The Uses of Hexetidine
Involved in studies:
- To identify pure liquid salt forms of aspirin
- Of alkyl based selective displacers for protein purification via ion exchange chromatography
- Of the in vivo application of models for testing drugs against nonreplicating Mycobacterium tuberculosis
- Nutrient-sensitized screening for drugs that shift the energy metabolism from mitochondrial respiration to glycolysis
- Inhibition of angiogensis
- Screening antimalarial drugs
Background
A bactericidal and fungicidal antiseptic. It is used as a 0.1% mouthwash for local infections and oral hygiene.
What are the applications of Application
Hexetidine, mixture of stereoisomers is an antibacterial and antifungal agent
Production Methods
Hexetidine is prepared by hydrogenation under pressure of 1,3- bis(2-ethylhexyl)-5-methyl-4-nitrohexahydropyriminine at 100°C using Raney nickel as a catalyst.
Definition
ChEBI: 1,3-bis(2-ethylhexyl)-5-methyl-1,3-diazinan-5-amine is an organonitrogen heterocyclic compound and an organic heteromonocyclic compound.
Manufacturing Process
Nitroethane and formaldehyde are first reacted to give 2-methyl-2-nitro-1,3-
propanediol. This is reacted with 2-ethylhexylamine and formaldehyde to give
5-nitro-1,3-bis(2-ethylhexyl)-5-methyl-hexahydropyrimidine.
To a hydrogenation apparatus containing 500 ml of methanol and 10 g of
Raney nickel catalyst were continuously added over a period of one hour, 240
g of 5-nitro-1,3-bis(2-ethylhexyl)-5-methylhexahydropyrimidine. During the
one-hour period, the resulting mixture was hydrogenated at approximately
1,000 pounds per square inch utilizing room temperature as the initial
temperature and gradually increasing the temperature to about 70°C. At the
end of the one-hour period, hydrogenation was stopped. The reaction mixture
was first filtered to remove the catalyst and was then distilled at atmospheric
pressure at a temperature of 70°C to remove methanol. 197.5 g of 5-amino-
1,3-bis(2-ethylhexyl)-5-methylhexahydropyrimidine were collected.
Therapeutic Function
Antifungal
General Description
The effectiveness of a hexetidine mouthwash in reducing supragingival plaque and gingival inflammation has been examined.
Hazard
Combustible.
Pharmaceutical Applications
Hexetidine is used as an antimicrobial preservative in cosmetics and nonparenteral pharmaceutical formulations. Therapeutically, hexetidine is mainly used as a 0.1% w/v solution in mouthwash formulations for the prevention and treatment of minor local infections, gingivitis, and mouth ulcers.
Safety
Hexetidine is mainly used in mouthwashes as a bactericidal and
fungicidal antiseptic. It is also used as an antimicrobial preservative
and is generally regarded as a relatively nontoxic and nonirritant
material at concentrations up to 0.1% w/v. Allergic contact
dermatitis and altered olfactory and taste perception have
occasionally been reported. Hexetidine is toxic when administered
intravenously.
Solutions of hexetidine in oil at concentrations of 5–10% w/v
cause strong primary irritations without sensitization in humans.
Long-term toxicological studies of up to 0.1% w/w of hexetidine in
food for 1 year do not show any toxic effect. Fetotoxicity,
embryotoxicity, and teratogenicity studies in rats of doses up to
50 mg/kg/day exhibit no sign of toxicity.
LD100 (cat, IV): 5–20 mg/kg
LD50 (dog, oral): 1.60 g/kg
LD50 (mouse, IP): 0.142 g/kg
LD50 (mouse, oral): 1.52 g/kg
LD50 (rat, oral): 0.61–1.43 g/kg
Metabolism
Not Available
storage
Hexetidine is stable and should be stored in a well-closed container in a cool, dry place. Brass and copper equipment should not be used for the handling or storage of hexetidine.
Incompatibilities
Hexetidine is incompatible with strong oxidizing agents. Salts are formed with mineral and organic acids; strong acids cause opening of the hexahydropyrimidine ring, releasing formaldehyde.
Regulatory Status
Included in nonparenteral formulations licensed in Europe.
Properties of Hexetidine
| Melting point: | 25°C |
| Boiling point: | 160 °C/0.4 mmHg (lit.) |
| Density | 0.889 g/mL at 25 °C (lit.) |
| refractive index | 1.4649 |
| Flash point: | 70°C |
| storage temp. | 2-8°C |
| solubility | acetone: soluble(lit.) |
| form | neat |
| pka | 8.3(at 25℃) |
| form | Liquid |
| color | Clear Colourless |
| Water Solubility | Not miscible or difficult to mix in water. |
| Merck | 14,4703 |
| BRN | 161071 |
| CAS DataBase Reference | 141-94-6(CAS DataBase Reference) |
| EPA Substance Registry System | 5-Pyrimidinamine, 1,3-bis(2-ethylhexyl)hexahydro-5-methyl- (141-94-6) |
Safety information for Hexetidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Hexetidine
| InChIKey | DTOUUUZOYKYHEP-UHFFFAOYSA-N |
Hexetidine manufacturer
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran



You may like
-
 141-94-6 Hexetidine 98%View Details
141-94-6 Hexetidine 98%View Details
141-94-6 -
 Hexetidine 97% CAS 141-94-6View Details
Hexetidine 97% CAS 141-94-6View Details
141-94-6 -
 Hexetidine CAS 141-94-6View Details
Hexetidine CAS 141-94-6View Details
141-94-6 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
