2-Aminophenol
Synonym(s):2-Aminophenol;2-Hydroxy aniline, 2-Amino-1-hydroxy benzene
- CAS NO.:95-55-6
- Empirical Formula: C6H7NO
- Molecular Weight: 109.13
- MDL number: MFCD00007690
- EINECS: 202-431-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
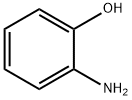
What is 2-Aminophenol?
Chemical properties
2-aminophenol appears as colorless needles or as white crystalline substance turning tan to brown on exposure to air. It forms white orthorhombic bipyramidal needles when crystallized from water or benzene. The crystals have eight molecules to the elementary cell. Insoluble in benzene, soluble in ethanol and water. o-aminophenol is contained in hair dyes and can cause contact dermatitis in hairdressers.
The Uses of 2-Aminophenol
2-Aminophenol is an isomer of 4-Aminophenol and is used as a reagent for the synthesis of heterocyclic compounds and dyes. It is also used to make dyes and pharmaceuticals.
Definition
ChEBI: 2-aminophenol is the aminophenol which has the single amino substituent located ortho to the phenolic -OH group. It has a role as a bacterial metabolite.
Preparation
2-Aminophenol is obtained by reduction of o-nitrophenol with sodium sulfide or hydrogen gas.
Synthesis Reference(s)
Synthetic Communications, 13, p. 495, 1983 DOI: 10.1080/00397918308081828
Antimicrobial activity
Further observations on this group of compounds are required, but its low toxicity and its bacteriostatic activity against the Gram-negative bacilli suggest that 2-aminophenol may be of value as a local antiseptic.
General Description
2-Aminophenol, also called o-aminophenol, is an aromatic amphoteric compound with the formula C6H4(OH)NH2. It is a white needle-shaped crystal that darkens when exposed to light and air. It is an isomer of aminophenol and serves as a vital chemical intermediate for dyes, medicine, printing, and biological applications.
Air & Water Reactions
Protect from air and light. Insoluble in water.
Reactivity Profile
2-Aminophenol can react with oxidizing agents. THF forms explosive products with 2-aminophenol [Lewis 3227].
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Contact allergens
It is contained in hair dyes and can cause contact dermatitis in hairdressers and consumers
Side Effects
(1)Methemoglobinemia - an increase in methemoglobin in the blood; the compound is classified as having secondary toxic effects.
(2)Skin sensitiser - a substance that can induce an allergic skin reaction.
(3)Asthma - reversible bronchoconstriction (narrowing of the fine bronchial tubes) caused by inhalation of irritating or allergenic substances.
Safety Profile
Poison by intraperitoneal and subcutaneous routes. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. An eye irritant. Mutation data reported. When heated to decomposition it emits toxic NO,. See also AROMATIC AMINES.
Toxicology
2-Aminophenol can act as a skin sensitiser and cause contact dermatitis. In addition, inhalation of large amounts can cause methemoglobinemia and bronchial asthma. Methemoglobinemia is the formation of methemoglobin when 2-aminophenol interacts with adult and fetal hemoglobin. Compared to its isomers 3-aminophenol and 4-aminophenol, 2-aminophenol is the most potent in forming methemoglobinemia. Since methemoglobin cannot bind oxygen as well as haemoglobin, this may lead to tissue hypoxia.
Synthesis
2-Aminophenol is industrially synthesized by reducing the corresponding nitrophenol by hydrogen in the presence of various catalysts. The nitrophenols can also be reduced with iron.
Potential Exposure
Workers may be exposed to oAminophenol during its use as a chemical intermediate; in the manufacture of azo and sulfur dyes; and in the photographic industry. There is potential for consumer exposure to o-Aminophenol because of its use in dyeing hair, fur, and leather. The compound is a constituent of 75 registered cosmetic products suggesting the potential for widespread consumer exposure. p-Aminophenol is used mainly as a dye, dye intermediate and as a photographic developer; and in small quantities in analgesic drug preparation. Consumer exposure to p-aminophenol may occur from use as a hairdye or as a component in cosmetic preparations. mAminophenol is used mainly as a dye intermediate
Shipping
UN2512 Aminophenols (o-; m-; p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Purify it by dissolving it in hot water, decolorising with activated charcoal, filtering and cooling to induce crystallisation. Maintain an atmosphere of N2 over the hot phenol solution to prevent its oxidation [Charles & Freiser J Am Chem Soc 74 1385 1952]. It can also be crystallised from EtOH using the same precautions. [Beilstein 13 IV 805.]
Incompatibilities
These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as alkali metals, hydrides, nitrides, and sulfides. Flammable gas (H2) may be generated, and the heat of the reaction may cause the gas to ignite and explode. Heat may be generated by the acidbase reaction with bases; such heating may initiate polymerization of the organic compound. Reacts with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (e.g., by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of 2-Aminophenol
| Melting point: | 172 °C |
| Boiling point: | 164 °C (11.2515 mmHg) |
| Density | 1.328 |
| vapor pressure | 14 hPa (153 °C) |
| refractive index | 1.5444 (estimate) |
| Flash point: | 168 °C |
| storage temp. | Store below +30°C. |
| solubility | 17 g/L (20°C) |
| pka | 4.78, 9.97(at 20℃) |
| form | Powder |
| Colour Index | 76520 |
| color | White to brown |
| PH | 6.5-7.5 (10g/l, H2O, 20℃) |
| PH Range | 6.5 - 7.5 at 10 g/l at 20 °C |
| Water Solubility | 17 g/L (20 ºC) |
| Merck | 14,461 |
| BRN | 606075 |
| Stability: | Stable, but may be air or light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 95-55-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2-amino-(95-55-6) |
| EPA Substance Registry System | o-Aminophenol (95-55-6) |
Safety information for 2-Aminophenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 2-Aminophenol
2-Aminophenol manufacturer
Molsyns Research
CEFA CILINAS BIOTICS PVT LTD
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
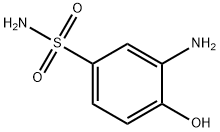
You may like
-
 2-Aminophenol 98%View Details
2-Aminophenol 98%View Details -
 2-Aminophenol 98%View Details
2-Aminophenol 98%View Details -
 2-Aminophenol CAS 95-55-6View Details
2-Aminophenol CAS 95-55-6View Details
95-55-6 -
 2-Aminophenol CAS 95-55-6View Details
2-Aminophenol CAS 95-55-6View Details
95-55-6 -
 2-Aminophenol 95-55-6 98%View Details
2-Aminophenol 95-55-6 98%View Details
95-55-6 -
 2-Aminophenol 99%View Details
2-Aminophenol 99%View Details -
 2-AMINOPHENOL Extra Pure CAS 95-55-6View Details
2-AMINOPHENOL Extra Pure CAS 95-55-6View Details
95-55-6 -
 2-Aminophenol CAS 95-55-6View Details
2-Aminophenol CAS 95-55-6View Details
95-55-6
