Hexamethyleneimine
Synonym(s):Azepane;HMI;Homopiperidine;Perhydroazepine;Hexahydro-1H-azepine
- CAS NO.:111-49-9
- Empirical Formula: C6H13N
- Molecular Weight: 99.17
- MDL number: MFCD00006934
- EINECS: 203-875-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
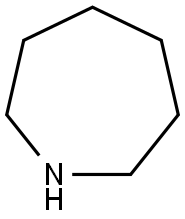
What is Hexamethyleneimine?
Chemical properties
clear colorless to light yellow liquid
The Uses of Hexamethyleneimine
Hexamethyleneimine is used as a pharmaceutical product and raw material for rubber products. It is also used as a intermediate for agrochemicals, zeolites, dyes , inks, rubber chemicals, textile chemicals, corrosion inhibitors, ore flotation.
The Uses of Hexamethyleneimine
A degradation product of herbicide Molinate in pot soil and rice plants.
Definition
ChEBI: An azacycloalkane that is cycloheptane in which one of the carbon atoms is replaced by a nitrogen atom.
Production Methods
Hexamethyleneimine is produced in 84% yield by heating hexamethylenediamine at 350 ℃ in a stream of hydrogen. The catalyst, ammonium vanadate on activated alumina, is prereduced with hydrogen at 500 ℃. Residues from the industrial distillation of hexamethylenediamine can be converted into hexamethyleneimine over aluminum silicate or aluminum oxide in a stream of nitrogen. For the preparation from caprolactam]. Hexamethyleneimine can be prepared by dimerizing acrolein, reducing the product to 2- hydroxymethyltetrahydropyran, expanding the ring to give oxepane, followed by treatment with ammonia over aluminum oxide at 350 ℃.
What are the applications of Application
The most important use is the conversion of hexamethyleneimine into S-ethylhexahy- dro-1H-azepine-1-carbothioate, the selective rice herbicide Molinate (C2H5 S–CO–NC6H12 , Zeneca).
General Description
A colorless liquid with an ammonia-like odor. Flash point 65°F. Toxic by ingestion. Corrosive to metals and tissue. Combustion produces toxic oxides of nitrogen.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Hexamethyleneimine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides.
Hazard
Toxic by ingestion, strong irritant to tissue.
Health Hazard
Inhalation of vapor irritates respiratory tract; high concentrations may cause disturbance of the central nervous system. Ingestion causes burns of mouth and stomach. Contact with concentrated vapor may cause severe eye injury. Contact with liquid causes burns of eyes and skin.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Purification Methods
Purify azepane by dissolving in Et2O and adding ethanolic HCl until all the base separates as the white hydrochloride, filter, wash with Et2O and dry it (m 236o). The salt is dissolved in the minimum volume of H2O and basified to pH ~ 14 with 10N KOH. The solution is extracted with Et2O, the extract is dried over KOH, evaporated and distilled. The free base is a FLAMMABLE and TOXIC liquid, and best kept as the salt. The nitrate has m 120-123o, the picrate has m 145-147o, and the tosylate has m 76.5o (ligroin). [Müller & Sauerwald Monatsh Chem 48 727 1027, Hjelt & Agback Acta Chem Scand 18 194 1964, Beilstein 20 II 1406, 20 III/IV 1406, 20/4 V 3.]
Properties of Hexamethyleneimine
| Melting point: | -37°C |
| Boiling point: | 138 °C749 mm Hg(lit.) |
| Density | 0.88 g/mL at 25 °C(lit.) |
| vapor pressure | 7.4 mm Hg ( 21.1 °C) |
| refractive index | n |
| Flash point: | 65 °F |
| storage temp. | Poison room |
| form | Liquid |
| pka | pK1:11.07 (25°C) |
| color | Clear colorless to light yellow |
| Odor | Ammonia-like. |
| explosive limit | 1.6-9.9%(V) |
| Water Solubility | soluble |
| BRN | 1084 |
| CAS DataBase Reference | 111-49-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Azepine, hexahydro-(111-49-9) |
| EPA Substance Registry System | 1H-Azepine, hexahydro- (111-49-9) |
Safety information for Hexamethyleneimine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H300:Acute toxicity,oral H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Hexamethyleneimine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[(2-Chlorophenyl)-N-(methylimino)methyl]cyclopentanol hydrochloride](https://img.chemicalbook.in/CAS/GIF/90717-16-1.gif)
You may like
-
 Hexamethyleneimine 95% CAS 111-49-9View Details
Hexamethyleneimine 95% CAS 111-49-9View Details
111-49-9 -
 Hexamethyleneimine CAS 111-49-9View Details
Hexamethyleneimine CAS 111-49-9View Details
111-49-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1