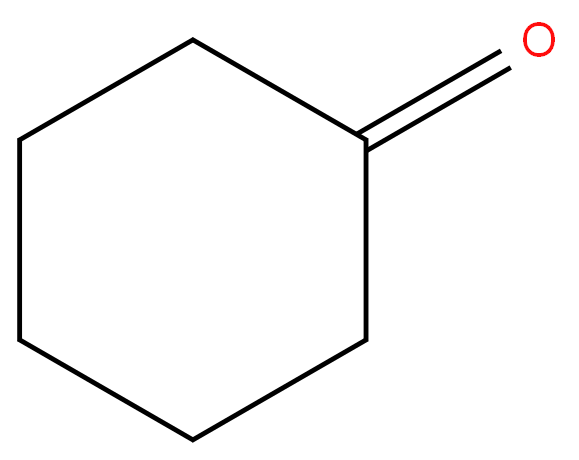
Cyclohexanone
Synonym(s):Cyclohexanone;Pimelic ketone
- CAS NO.:108-94-1
- Empirical Formula: C6H10O
- Molecular Weight: 98.14
- MDL number: MFCD00001625
- EINECS: 203-631-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:09
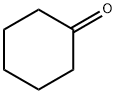
What is Cyclohexanone?
Description
Cyclohexanone (C6H10O), a colorless liquid is a cyclic ketone with a minty or acetone-like odor. It is used as a solvent and as a precursor in the manufacturing of nylon.
Chemical properties
Cyclohexanone is a water-white to slightly yellow liquid with a peppermint-like or acetone-like odor. The Odor Threshold is 0.12 0.24 ppm in air.
Physical properties
Clear, colorless to pale yellow, oily liquid with a peppermint-like odor. Experimentally determined detection and recognition odor threshold concentrations were identical: 480 μg/m3 (120 ppmv) (Hellman and Small, 1974).
The Uses of Cyclohexanone
Cyclohexanone is used in the productionof adipic acid for making nylon; in thepreparation of cyclohexanone resins; and asa solvent for nitrocellulose, cellulose acetate,resins, fats, waxes, shellac, rubber, and DDT. Further, it is used as a chemical reaction medium, adhesives, sealants and agricultural products.
Definition
ChEBI: Cyclohexanone is a cyclic ketone that consists of cyclohexane bearing a single oxo substituent. It has a role as a human xenobiotic metabolite.
Synthesis Reference(s)
Canadian Journal of Chemistry, 62, p. 1031, 1984 DOI: 10.1139/v84-171
Tetrahedron Letters, 25, p. 3309, 1984 DOI: 10.1016/S0040-4039(01)81371-X
Air & Water Reactions
Flammable. Soluble in water.
Reactivity Profile
Cyclohexanone forms an explosive peroxide with H2O2, and reacts vigorously with oxidizing materials (nitric acid).
Toxicity
The toxicity of cyclohexanone in test specieswas found to be low to moderate. Exposureto its vapors can produce irritation in the eyesand throat. Splashing into the eyes can damagethe cornea. Throat irritation in humansmay occur from 3–5 minute exposure to a50-ppm concentration in air. The symptomsof chronic toxicity in animals from its inhalationwere liver and kidney damage, as wellas weight loss. However, its acute toxicitywas low below 3000 ppm. The symptomsin guinea pigs were lacrimation, salivation,lowering of heart rate, and narcosis. Exposureto 4000 ppm for 4–6 hours was lethalto rats and guinea pigs.
The oral toxicity of this compound waslow. Ingestion may cause narcosis and depressionof the central nervous system. It canbe absorbed through the skin.
LD50 value, dermal (rabbits): 1000 mg/kg
LD50 value, intraperitoneal (rats): 1130 mg/kg.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Contact allergens
Used as a polyvinyl chloride solvent, cyclohexanone caused contact dermatitis in a woman manufacturing PVC fluidotherapy bags. Cyclohexanone probably does not cross-react with cyclohexanone resin. A cyclohexanone-derived resin used in paints and varnishes caused contact dermatitis in painters
Safety Profile
Suspected carcinogen. Moderately toxic by ingestion, inhalation, subcutaneous, intravenous, and intraperitoneal routes. A skin and severe eye irritant. Human systemic effects by inhalation: changes in the sense of smell, conjunctiva irritation, and unspecified respiratory system changes. Human irritant by inhalation. Mdd narcotic properties have also been ascribed to it. Human mutation data reported. Experimental reproductive effects. Flammable liquid when exposed to heat or flame; can react vigorously with oxidizing materials. Slight explosion hazard in its vapor form, when exposed to flame.Reaction with hydrogen peroxide + nitric acid forms an explosive peroxide. To fight fire, use alcohol foam, dry chemical, or COa. When heated to decomposition it emits acrid smoke and irritating fumes. See also KETONES and CYCLOHEXANE.
Potential Exposure
May form explosive mixture with air. Contact with oxidizing agents or nitric acid may cause a violent reaction. Do not use brass, copper, bronze, or lead fittings. Attacks many coatings and plastic materials.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
IARC considers the animal data for cyclohexanone as inadequate evidence of carcinogenicity and listed cyclohexanone as not classifiable for carcinogenicity (IARC Category 3).
Environmental Fate
Biological. In activated sludge inoculum, 96.0% COD removal was achieved. The average rate
of biodegradation was 30.0 mg COD/g?h (Pitter, 1976).
Photolytic. Atkinson (1985) reported an estimated photooxidation rate constant of 1.56 x 10-11
cm3/molecule?sec for the reaction of cyclohexanone and OH radicals in the atmosphere at 298 K.
Chemical/Physical. Cyclohexanone will not hydrolyze because it has no hydrolyzable
functional group.
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in effluent
concentration of 332 mg/L. The adsorbability of the carbon used was 134 mg/g carbon (Guisti et
al., 1974). Similarly, at influent concentrations of 10, 1.0, 0.1, and 0.01 mg/L, the GAC adsorption
capacities were 36, 6.2, 1.1, and 0.19 mg/g, respectively (Dobbs and Cohen, 1980).
Storage
Cyclohexanone must be stored to avoid contact with oxidizers (such as perchlorates, peroxides, chlorates, nitrates, andpermanganates), since violent reactions occur. Store intightly closed containers in a cool well-ventilated area awayfrom heat, sparks, and flames.
Wherever this chemicalis used, handled, manufactured, or stored, use explosionproof electrical equipment and fittings.Cyclohexanone requires a “FLAMMABLELIQUID” label. It falls in Hazard Class 3 and PackingGroup II
Shipping
UN1915 Cyclohexanone, Hazard Class: 3; Labels: 3-Flammable liquid.
Incompatibilities
May form explosive mixture with air. Contact with oxidizing agents or nitric acid may cause a violent reaction. Do not use brass, copper, bronze, or lead fittings. Attacks many coatings and plastic materials.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Cyclohexanone
| Melting point: | -47 °C (lit.) |
| Boiling point: | 155 °C (lit.) |
| Density | 0.947 g/mL at 25 °C (lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 3.4 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3909 | CYCLOHEXANONE |
| Flash point: | 116 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 90g/l |
| form | Liquid |
| pka | 17(at 25℃) |
| color | APHA: ≤10 |
| Odor | Like peppermint and acetone. |
| Relative polarity | 0.281 |
| PH | 7 (70g/l, H2O, 20℃) |
| explosive limit | 1.1%, 100°F |
| Water Solubility | 150 g/L (10 ºC) |
| Merck | 14,2726 |
| JECFA Number | 1100 |
| BRN | 385735 |
| Henry's Law Constant | 1.2 x 10-5 atm?m3/mol at 25 °C (Hawthorne et al., 1985)
6.92 x 10-5 atm?m3/mol at 60.00 °C, 10.7 at 70.00 °C, 16.4 at 80.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | TLV-TWA 100 mg/m3 (25 ppm) (ACGIH);
IDLH 5000 ppm (NIOSH). |
| Dielectric constant | 18.2(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 108-94-1(CAS DataBase Reference) |
| IARC | 3 (Vol. 47, 71) 1999 |
| NIST Chemistry Reference | Cyclohexanone(108-94-1) |
| EPA Substance Registry System | Cyclohexanone (108-94-1) |
Safety information for Cyclohexanone
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cyclohexanone
Cyclohexanone manufacturer
Suraj Enterprises
Gayatri Industries
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cyclohexanone 99%View Details
Cyclohexanone 99%View Details -
 Cyclohexanone 98%View Details
Cyclohexanone 98%View Details -
 Cyclo Hexanone 99%View Details
Cyclo Hexanone 99%View Details -
 Cyclohexanone, For ACS analysis CAS 108-94-1View Details
Cyclohexanone, For ACS analysis CAS 108-94-1View Details
108-94-1 -
 Cyclohexanone, For ACS analysis CAS 108-94-1View Details
Cyclohexanone, For ACS analysis CAS 108-94-1View Details
108-94-1 -
 Cyclohexanone, For ACS analysis CAS 108-94-1View Details
Cyclohexanone, For ACS analysis CAS 108-94-1View Details
108-94-1 -
 Cyclohexanone, For ACS analysis CAS 108-94-1View Details
Cyclohexanone, For ACS analysis CAS 108-94-1View Details
108-94-1 -
 Cyclohexanone CASView Details
Cyclohexanone CASView Details
