Methylene Blue trihydrate
Synonym(s):Methylene blue;Methylthioninium chloride;Methylene Blue trihydrate;Methylene Blue hydrate;Methylthionini chloridum
- CAS NO.:7220-79-3
- Empirical Formula: C16H20ClN3OS
- Molecular Weight: 337.87
- MDL number: MFCD00150008
- EINECS: 615-731-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-29 11:36:03
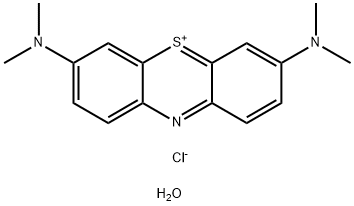
What is Methylene Blue trihydrate?
Chemical properties
Methylene Blue trihydrate is a dark green crystalline powder that has a bronze-like luster. When dissolved in water or alcohol, it creates a deep blue color. Methylene blue is used as a bacteriologic stain and as an indicator. It can also inhibit guanylate cyclase and has been used to treat cyanide poisoning and lower levels of methemoglobin.
The Uses of Methylene Blue trihydrate
Methylene Blue trihydrate finds many uses in biology and chemistry. It acts as a GCS inhibitor, biological stain and redox indicator. It inhibits tau filament formation. Further, it is reported to bind to the M2 subtype of muscarinic receptors in rat cardiac myocytes and thus exhibit antimuscarinic effects on IK, ACh and ICa. It also inhibits endothelium-dependent relaxation of isolated blood vessels.
What are the applications of Application
Methylene blue trihydrate is an inhibitor of cGMP-mediated processes and guanylyl cyclase, a useful stain, and a redox indicator
What are the applications of Application
Methylene blue is not an officially restricted drug. It is mainly used to control water mildew and small melon insects. It can replace malachite green. Methylene blue dissolved in water does little harm to fish. In addition, the drug has made achievements in many fields such as industry, medicine and skin care. Even the drug will be applied to more difficult cancer diseases. Methylene blue is more effective in the treatment of non advanced fish diseases. Methylene blue is an oxidant, which can oxidize nitrite into nitrate, inhibit and kill bacteria. However, it also has a certain impact on nitrifying bacteria, so some measures should be taken after use.
Methylene blue can be used in the manufacture of ink and lakes and the dyeing of biological and bacterial tissues. It can be used for dyeing cotton, hemp, silk fabrics, paper and bamboo and wood. It can also be mixed with crystal violet and yellow dextrin in the ratio of 78:13:9 to form alkaline magenta blue.
Preparation
Methylene blue is synthesized commercially by oxidation of N,N-dimethyl-phenylenediamine with sodium dichromate (Na2Cr2O7) in the presence of sodium thiosulfate (Na2S2O3), followed by further oxidation in the presence of N,N-dimethylaniline (NTP, 2008). Methylene blue hydrochloride is isolated by addition of 30% hydrochloric acid and of a saturated common salt solution to the dye solution; after filtration, the product is washed with a 2% common salt solution. Instead of sodium dichromate, manganese dioxide, and catalytic amounts of copper sulfate can be used for the oxidation (Berneth, 2008).
Methylene blue of high purity can be obtained by chloroform extraction of impurities from solutions of raw dye in borate buffer at pH 9.5–10, followed by acidification of the aqueous solution and isolation of the dye (Berneth, 2008).
IARC Monographs
General Description
Methylene blue trihydrate appears as odorless or almost odorless dark green crystals with bronze luster or dark green crystalline powder. pH (1% solution in water) 3 to 4.5. (NTP, 1992)
Air & Water Reactions
Hygroscopic. Water soluble. Gives a blue solution in water.
Reactivity Profile
Methylene Blue trihydrate is incompatible with strong oxidizing agents, caustics, alkali metal iodides and reducing agents. Also incompatible with dichromates. Methylene Blue trihydrate forms double salts with many inorganic salts. Is bleached reversibly by (zinc + hydrochloric acid) or sodium hydrosulfite. In aqueous solution, Methylene Blue trihydrate is decolorized by zinc dust and dilute sulfuric acid, but the color is restored upon exposure to air and more rapidly upon addition of ammonium hydroxide.
Fire Hazard
Flash point data for Methylene Blue trihydrate are not available; however, Methylene Blue trihydrate is probably combustible.
Storage
Room temperature
Properties of Methylene Blue trihydrate
| Melting point: | 190 °C (dec.)(lit.) |
| Density | 0.98 g/mL at 25 °C |
| Flash point: | 14 °C |
| storage temp. | no restrictions. |
| solubility | Soluble in water, chloroform; sparingly soluble in ethanol |
| form | Solid |
| pka | 2.6, 11.2(at 25℃) |
| Colour Index | 52015 |
| color | Green |
| Water Solubility | 4 g/100 mL |
| λmax | 668 nm, 609 nm |
| Merck | 13,6085 |
| Stability: | Stable. Incompatible with bases, reducing agents, strong oxidizing agents. |
| Biological Applications | Diagnosis of tauopathy,Alzheimer’s disease (AD),Pick’s disease; treating prophylaxis; dental materials |
| CAS DataBase Reference | 7220-79-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 108) 2016 |
| EPA Substance Registry System | Methylene blue trihydrate (7220-79-3) |
Safety information for Methylene Blue trihydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Methylene Blue trihydrate
| InChIKey | YAFGUSOAMIMROI-UHFFFAOYSA-M |
Methylene Blue trihydrate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 7220-79-3 METHYLENE BLUE 99%View Details
7220-79-3 METHYLENE BLUE 99%View Details
7220-79-3 -
 7220-79-3 99%View Details
7220-79-3 99%View Details
7220-79-3 -
 Methylene Blue trihydrate 7220-79-3 98%View Details
Methylene Blue trihydrate 7220-79-3 98%View Details
7220-79-3 -
 7220-79-3 Methylene blue trihydrate 99%View Details
7220-79-3 Methylene blue trihydrate 99%View Details
7220-79-3 -
 Methylene Blue CAS 7220-79-3View Details
Methylene Blue CAS 7220-79-3View Details
7220-79-3 -
 Methylene Blue Trihydrate CAS 7220-79-3View Details
Methylene Blue Trihydrate CAS 7220-79-3View Details
7220-79-3 -
 Methylene blue (C.I. 52015) CAS 7220-79-3View Details
Methylene blue (C.I. 52015) CAS 7220-79-3View Details
7220-79-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
