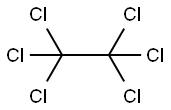
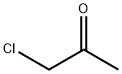
Hexachloroacetone
Synonym(s):HCA;Hexachloroacetone
- CAS NO.:116-16-5
- Empirical Formula: C3Cl6O
- Molecular Weight: 264.75
- MDL number: MFCD00000796
- EINECS: 204-129-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
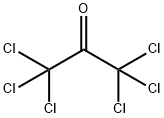
What is Hexachloroacetone?
Chemical properties
clear colorless to yellowish liquid
The Uses of Hexachloroacetone
Hexachloro-2-propanone (hexachloroacetone) was used in the synthesis of chirally deuterated benzyl chlorides and enol phosphate 2,2-dichloro-1-(trichloromethyl)ethenyl diethyl phosphate. It was also used in obtaining efficient amino acid activation by exploiting the rapid formation of acid chlorides under low temperature and acid/base free conditions.
The Uses of Hexachloroacetone
Reagent used for the polymerization of vinyl compounds and for the conversion of nucleoside-3′-phosphonates to the corresponding phosphates
What are the applications of Application
Hexachloro-2-propanone is Reagent used for the polymerization of vinyl compounds and for the conversion of nucleoside-3′-phosphonates to the corresponding phosphates
Definition
ChEBI: Hexachloroacetone is a ketone.
General Description
A yellow-colored liquid. Slightly soluble in water and denser than water. Vapors are much heavier than air. Irritates skin and eyes. May be toxic by ingestion or inhalation. Used to make other chemicals.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Hexachloroacetone is a halogenated ketone. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Hazard
Toxic by ingestion and inhalation, strong irritant. Combustible. Evolves phosgene when heated.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Properties of Hexachloroacetone
| Melting point: | -3 °C |
| Boiling point: | 66-70 °C6 mm Hg(lit.) |
| Density | 1.743 g/mL at 25 °C(lit.) |
| vapor pressure | 10-130Pa at 20-50℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Water Solubility | SLIGHTLY SOLUBLE |
| BRN | 1707475 |
| Dielectric constant | 3.9900000000000002 |
| CAS DataBase Reference | 116-16-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanone, 1,1,1,3,3,3-hexachloro-(116-16-5) |
| EPA Substance Registry System | Hexachloroacetone (116-16-5) |
Safety information for Hexachloroacetone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H331:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Hexachloroacetone
| InChIKey | DOJXGHGHTWFZHK-UHFFFAOYSA-N |
Hexachloroacetone manufacturer
JSK Chemicals
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Hexachloroacetone CAS 116-16-5View Details
Hexachloroacetone CAS 116-16-5View Details
116-16-5 -
 Hexachloroacetone CAS 116-16-5View Details
Hexachloroacetone CAS 116-16-5View Details
116-16-5 -
 Hexachloroacetone CAS 116-16-5View Details
Hexachloroacetone CAS 116-16-5View Details
116-16-5 -
 Hexachloro-2-propanone CAS 116-16-5View Details
Hexachloro-2-propanone CAS 116-16-5View Details
116-16-5 -
 Hexachloroacetone CAS 116-16-5View Details
Hexachloroacetone CAS 116-16-5View Details
116-16-5 -
 116-16-5 98%View Details
116-16-5 98%View Details
116-16-5 -
 Hexachloroacetone, 98% 116-16-5 99%View Details
Hexachloroacetone, 98% 116-16-5 99%View Details
116-16-5 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
