Hexachloroethane
Synonym(s):Hexachloroethane solution;Perchloroethane
- CAS NO.:67-72-1
- Empirical Formula: C2Cl6
- Molecular Weight: 236.74
- MDL number: MFCD00000799
- EINECS: 200-666-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
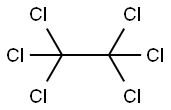
What is Hexachloroethane?
Description
Hexachloroethane (HCE) is a halogenated hydrocarbon consisting
of six chlorines attached to an ethane (ACGIH, 1991); it
is a white to pale yellow solid that is unstable in air and evaporates
gradually. It smells like camphor when its concentration
in air and water are 150 and 10 ppb, respectively. HCE itself
does not catch fire easily; however; in aqueous nonbiological
conditions it has been determined that HCE is unstable and
nonenzymatic dechlorination in the absence of nicotinamide
adenine dinucleotide phosphate (NADP) occurs. It rapidly
degrades in soil or groundwater. Also, some microorganisms
break down HCE without oxygen, and decomposition in
aerobic conditions has been reported. Some bioconcentration
of HCE in fish has been determined, though upper levels
through the food chain are limited, since it is rapidly metabolized
by fish, which is discussed later (ATSDR, 1997).
Eyes, skin, respiratory system, and kidneys have been
proposed as main targets in humans upon exposure. Symptoms
include blinking, tearing, photophobia, and irritation of
eyes. Also, facial muscles may have difficulty in movement.
Animal studies on effects of HCE during pregnancy are limited.
After oral exposure, HCE is primarily distributed to fat tissue.
Toxicokinetic studies in animals indicated that HCE is mostly
localized and metabolized in the liver and kidney. Several
corresponded metabolites have demonstrated liver and kidney
toxicities similar to HCE. Neurological effects such as tremors
and ataxia were observed in Beagle dogs, rats, and pregnant
rats. Other effects via inhalation exposure included reduced
body weight and increased relative liver weight in rats and
guinea pigs. In another study, male rats also displayed
increased relative spleen and testes weight. Based on California
Proposition 65, HCE was proposed to be carcinogenic for
humans, and it induces tumors at sites other than the site of
entry. Noncancerous effects include kidney degeneration
(tubular nephropathy, necrosis of renal tubular epithelium,
hyaline droplet formation, tubular regeneration, and tubular
casts) and hepatocellular necrosis. It results in hyaline droplet
nephropathy and renal toxicity, and it induces chromosome
malsegregation, lethality, and mitotic growth arrest (Crebelli
et al., 1995, 1992, 1988).
Chemical properties
white crystalline powder
Chemical properties
Hexachloroethane is a white solid with a camphor-like odor. It gradually evaporates when it is exposed to air.
Physical properties
Rhombic, triclinic or cubic, colorless crystals with a camphor-like odor. Odor threshold concentration is 0.15 ppm (quoted, Amoore and Hautala, 1983).
The Uses of Hexachloroethane
Hexachloroethane is used as a solvent, infireworks and smoke devices; in explosives,in celluloid, as an insecticide, and as a rubbervulcanizing accelerator. Earlier it was used asan anthelmintic for livestock. Hexachloroethane is a highly efficient chlorinating agent in the preparation of chlorosilanes from hydrosilanes.
The Uses of Hexachloroethane
In metallurgy for refining aluminum alloys, removing impurities from molten metals, recovering metal from ores or smelting products. Degassing agent for magnesium; to inhibit explosiveness of methane and combustion of ammonium perchlorate. Smoke generator in grenades; in pyrotechnics. Ignition suppressant, in fire extinguishing fluids, polymer additive, flame-proofing agent, vulcanizing agent. In production of synthetic diamonds.
The Uses of Hexachloroethane
The applications of hexachloroethane have been extensive; however, industrial uses are diminishing. Hexachloroethane is used primarily in military smoke munitions (e.g., smoke pots, grenades, cartridges, and projectiles used to generate “smoke” or “fog”) and in pyrotechnics.
The estimated average annual use of hexachloroethane from 1966 to 1977 at a major facility manufacturing smoke and pyrotechnic devices was 192,802 lb. In the 1970s, about half of the hexachloroethane distributed was used to manufacture military smoke-producing and pyrotechnic devices, 30% to 40% to manufacture degassing pellets to remove air bubbles from molten ore at aluminum foundries, and 10% to 20% as an antihelminthic to control liver flukes in sheep and cattle. The U.S. Food and Drug Administration withdrew approval for the use of hexachloroethane as an antihelminthic in 1971, and it probably is no longer used for this purpose (ATSDR 1997). Its use for degassing aluminum also has been almost completely phased out in the United States (EPA 1999). Other uses in metallurgy include refining alloys, removing impurities from molten metals, recovering metals from ores or smelting products, and as a degassing agent for magnesium; however, the European Union began phasing out the use of hexachloroethane in nonferrous metals in 1998 (EC 1998).
A number of other past uses of hexachloroethane have been identified, but many of these likely have been discontinued or involve the use of only limited quantities. Hexachloroethane is used as a laboratory chemical and as an ingredient in various fungicidal and insecticidal formulations, extreme-pressure lubricants, and plastics (ATSDR 1997, IARC 1999, HSDB 2009). Other past uses include as a moth repellent and in the chemical industry as a polymer additive, a plasticizer for cellulose esters, an accelerator, a vulcanizing agent, a process solvent in rubber manufacturing, a retardant in fermentation processes, and a component of submarine paints, and in the production of some types of synthetic diamonds. It has also been used as a component of fire-extinguishing fluids, an additive in combustible liquids (ignition suppressant), and an inhibitor of the explosiveness of methane and the combustion of ammonium perchlorate (IARC 1979, 1999, HSDB 2009).
Definition
ChEBI: A member of the class of chloroethanes that is ethane in which all the hydrogens are replaced by chloro groups.
General Description
Hexachloroethane is a colorless, crystalline solid with a camphor-like odor. Hexachloroethane may cause illness from inhalation or ingestion and may irritate skin, eyes and mucous membranes. When heated to high temperatures Hexachloroethane may emit toxic fumes. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Hexachloroethane is used to make other chemicals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Hexachloroethane can react with hot iron, zinc and aluminum. Dehalogenation of Hexachloroethane by reaction with alkalis and metals will produce unstable chloroacetylenes. Hexachloroethane can also react with strong oxidizing agents. .
Hazard
Toxic by ingestion and inhalation, strong irritant, absorbed by skin. Possible carcinogen.
Health Hazard
Compound is a powerful narcotic and liver poison; may also cause changes in blood composition and neurological disturbances. Repeated exposure by inhalation can be fatal. Ingestion causes vomiting, diarrhea, severe mucosal injury, liver necrosis, cyanosis, unconsciousness, loss of reflexes, and death. Contact with eyes causes irritation and lachrymation. Can be absorbed through the skin and may produce severe skin lesions.
Health Hazard
Vapors of hexachloroethane are an irritant tothe eyes and mucous membranes. Oral dosesof 1000 mg/kg produced weakness, stagger ing gait, and twitching muscles in dogs.Rabbits fed 1000 mg/kg for 12 days devel oped necrosis; a lower amount, 320 mg/kg,caused liver degeneration; no effects wereobserved at a dose level of 100 mg/kg(Weeks 1979).
Acute inhalation toxicity is of a loworder in animals. Subacute toxic effectsin dogs exposed to 260-ppm vapors ofhexachloroethane for 6 hours per day, 5days a week for 6 weeks were tremors,ataxia, hypersalivation, head bobbling, andfacial muscular fasciculations (Weeks 1979).The lethal concentration in rats is 5900 ppmfrom an 8-hour exposure.
LD50 value, oral (rats): 4460 mg/kg
Tests for mutagenicity and teratogenic ity were negative. The carcinogenic poten tial of hexachloroethane was noted in testanimals only at extremely heavy dosagesgiven continuously for a long period of time(ACGIH 1986). It caused liver tumors inmice.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride vapor may form in fire.
Potential Exposure
In the US, about half the HCE is used by the military for smoke-producing devices. It is also used to remove air bubbles in melted aluminum. It may be present as an ingredient in some fungicides, insecticides, lubricants, and plastics. It is no longer made in the United States, but it is formed as a by-product in the production of some chemicals. Can be formed by incinerators when mate rials containing chlorinated hydrocarbons are burned. Some HCE can also be formed when chlorine reacts with carbon compounds in drinking water. As a medicinal, HCE is used as an anthelmintic to treat fascioliasis in sheep and cattle. It is also added to the feed of ruminants, preventing metha nogenesis and increasing feed efficiency. HCE is used in metal and alloy production, mainly in refining aluminum alloys. It is also used for removing impurities from molten metals, recovering metals from ores or smelting products and improving the quality of various metals and alloys. HCE is contained in pyrotechnics. It inhibits the explosive ness of methane and the combustion of ammonium perchlo rate. Smoke containing HCE is used to extinguish fires. HCE has various applications as a polymer additive. It has flameproofing qualities, increases sensitivity to radiation crosslinking, and is used as a vulcanizing agent. Added to polymer fibers, HCE acts as a swelling agent and increases affinity for dyes.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once andirrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immedi-ately with soap and water. Seek medical attention immedi-ately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precau-tions,includingIresuscitation1 mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quanti-ties of water and induce vomiting. Do not make an uncon-scious person vomit.
Carcinogenicity
Hexachloroethane is reasonably anticipated to be ogen based on sufficient evidence of carcinogenicit a human carciny from studies in experimental animals.
Environmental Fate
Biological. Under aerobic conditions or in experimental systems containing mixed cultures,
hexachloroethane was reported to degrade to tetrachloroethane (Vogel et al., 1987). In an
uninhibited anoxic-sediment water suspension, hexachloroethane degraded to tetrachloroethylene.
The reported half-life for this transformation was 19.7 min (Jafvert and Wolfe, 1987). When
hexachloroethane (5 and 10 mg/L) was statically incubated in the dark at 25 °C with yeast extract
and settled domestic wastewater inoculum for 7 d, 100% biodegradation with rapid adaptation was
observed (Tabak et al., 1981).
Photolytic. When an aqueous solution containing hexachloroethane was photooxidized by UV
light at 90–95 °C, 25, 50, and 75% degraded to carbon dioxide after 25.2, 93.7, and 172.0 h,
respectively (Knoevenagel and Himmelreich, 1976).
Chemical/Physical. The reported hydrolysis half-life at 25 °C and pH 7 is 1.8 x 109 yr (Jeffers et
al., 1989). No hydrolysis was observed after 13 d at 85 °C and pH values of 3, 7, and 11 (Ellington
et al., 1987). Similarly, no measureable hydrolysis was observed under neutral and alkaline
conditions (Jeffers and Wolfe, 1996).
Storage
Color Code- Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Hexachloroethane must be stored to avoid contactwith hot iron, zinc, aluminum, and alkalis since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area away from heat. A regulated, markedarea should be established where this chemical is handled,used, or stored in compliance with OSHA Standard1910.1045.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Steam distil it, then crystallise it from 95% EtOH. Dry it in the dark under vacuum. [Beilstein 1 IV 148.]
Toxicity evaluation
Reports on human health effects are limited and confounded
by coexposure to multiple solvents or other toxicants (e.g.,
HCE-zinc oxide smoke), and are too small to provide definitive
conclusions on health effects. Animal studies suggest that HCE
is primarily metabolized to tetrachloroethylene (PERC) and
pentachloroethane by CYP450 enzymes of the liver, with likely
subsequent metabolism to TCE. Metabolites identified in the
urine include TCA, trichloroethanol, oxalic acid, dichloroethanol,
dichloroacetic acid, and monochloroacetic acid
(Gorzinski et al., 1985).
Studies of TCA (a potential metabolite of HCE) indicate
that free-radical generation may play a role in mediating
toxicity, particularly in the liver. However, no data are available
demonstrating generation of free radicals following exposure to
HCE, and it is unknown whether unchanged HCE or its
metabolites are responsible for the liver and kidney toxicities
observed in animal studies. Lipid peroxidation was reported by
formation of malondialdehyde and conjugated dienes, which
involves free radicals (Town et al., 1984). In another study, the
presence of radiolabeled carbon measured by in vivo binding
studies suggested that HCE can bind to DNA, RNA, and
protein. Therefore, renal toxicity and hepatotoxicity may also
involve HCE binding to DNA, RNA, or protein, resulting in
cytotoxicity and contributing to the cytotoxic damage from
radicals. Another hypothesis is the data that a α2u-globulin
mode of action could contribute to HCE-induced nephropathy
but they are not sufficient.
Incompatibilities
Incompatible with strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from strong bases.
Waste Disposal
Incineration after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids pro duced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must con form to EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Hexachloroethane
| Melting point: | 183-185 °C (dec.) (lit.) |
| Boiling point: | 186℃ |
| Density | 2.091 g/mL at 25 °C (lit.) |
| vapor density | 8.16 (vs air) |
| vapor pressure | 0.4 mm Hg ( 20 °C) |
| refractive index | 1.5282 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol, benzene, chloroform, ether |
| form | Crystals or Crystalline Powder |
| color | White |
| Water Solubility | 0.05 g/L (22 ºC) |
| Merck | 14,4679 |
| BRN | 1740341 |
| Henry's Law Constant | 1.43, 2.81, and 5.31 at 10, 20, and 30 °C, respectively (Munz and Roberts, 1987) |
| Exposure limits | TLV-TWA 10 ppm (~100 mg/m3) (ACGIH),
1 ppm (MSHA and OSHA), Lowest Feasi ble Limit (NIOSH); carcinogenicity: Animal
Limited Evidence (IARC). |
| Stability: | Stable. Non-combustible. May react with hot metals, strong oxidizing agents. |
| CAS DataBase Reference | 67-72-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, hexachloro-(67-72-1) |
| IARC | 2B (Vol. 73) 1999 |
| EPA Substance Registry System | Hexachloroethane (67-72-1) |
Safety information for Hexachloroethane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Hexachloroethane
Hexachloroethane manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 Hexachloroethane CAS 67-72-1View Details
Hexachloroethane CAS 67-72-1View Details
67-72-1 -
 Hexachloroethane 95% CAS 67-72-1View Details
Hexachloroethane 95% CAS 67-72-1View Details
67-72-1 -
 Hexachloroethane pure CAS 67-72-1View Details
Hexachloroethane pure CAS 67-72-1View Details
67-72-1 -
 Hexachloro ethane CAS 67-72-1View Details
Hexachloro ethane CAS 67-72-1View Details
67-72-1 -
 Hexachloroethane 99.00% CAS 67-72-1View Details
Hexachloroethane 99.00% CAS 67-72-1View Details
67-72-1 -
 Hexachloroethane Powder 99%, 99.90%View Details
Hexachloroethane Powder 99%, 99.90%View Details
67-72-1 -
 Highly Pure HexachloroethaneView Details
Highly Pure HexachloroethaneView Details
67-72-1 -
 HexachloroethaneView Details
HexachloroethaneView Details
67-72-1
