Chloroacetone
Synonym(s):1-Chloro-2-propanone;1-Chloropropan-2-one;Chloro-2-propanone;Chloroacetone;MCA
- CAS NO.:78-95-5
- Empirical Formula: C3H5ClO
- Molecular Weight: 92.52
- MDL number: MFCD00000936
- EINECS: 201-161-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
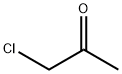
What is Chloroacetone?
Chemical properties
colourless to dark yellow liquid
The Uses of Chloroacetone
Chloroacetone was used in the synthesis of double-chain nonionic surfactants with an acid decomposition function via acid-catalyzed condensation with fatty alcohols (octyl, decyl and dodecyl). It was also used in the synthesis of meso-tetramethyl tetrakis-(4-phenoxy acetone)calix[4]pyrrole. It can also be used to make dye couplers for color photography and used in the Feist-Benary synthesis of furans.
The Uses of Chloroacetone
Couplers for color photography, enzyme inactivator, insecticides, perfumes, intermediate, organic synthesis, tear gas, polymerization of vinyl monomers.
The Uses of Chloroacetone
Manufacture of couplers for color photography; intermediate in manufacture of perfumes, antioxidants, drugs, plant growth regulators, defoliants, and herbicides
Definition
A lachrymator.
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 5274, 1955 DOI: 10.1021/ja01625a016
Synthesis, p. 188, 1987 DOI: 10.1055/s-1987-27886
General Description
A yellow-colored liquid with an irritating pungent odor. Light sensitive, but stabilized with the addition of small amounts of water and/or calcium carbonate. Slightly soluble in water and denser than water. Vapors much heavier than air. Irritates skin and eyes. Very toxic by ingestion or inhalation. Used to make other chemicals. A lachrymator.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
CHLOROACETONE turns dark and resinifies on prolonged exposure to light [Merck]. This occurred in a bottle during storage for two years on a shelf in diffused light. A few days after the bottle was moved, Chloroacetone exploded [Ind. Eng. News 9: 184(1931)]. Is stabilized by addition of 0.1% water or 0.1% CaCO3.
Hazard
Strong irritant to tissue, eyes, and mucous membranes; toxic by ingestion and skin contact. Upper respiratory tract irritant.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Safety Profile
Poison by inhalation, ingestion, and skin contact. Mutation data reported. A lachrymator poison gas. See also CHLORINATED HYDROCARBONS, ALIPHATIC; ACETONE. Flammable when exposed to heat or flame, or oxidizers. Old material can explode. When heated to decomposition it emits highly toxic fumes.
Purification Methods
Dissolve it in water and shake it repeatedly with small amounts of diethyl ether which extracts, preferentially, 1,1-dichloroacetone present as an impurity. The chloroacetone is then extracted from the aqueous phase using a large amount of diethyl ether, and distilled at slightly reduced pressure. It is dried with CaCl2 and stored at Dry-ice temperature. Alternatively, it was stood over CaSO4, distilled and stored over CaSO4. It is steam volatile. The 2,4-dinitrophenylhydrazone forms yellow needles from EtOH with m 120o or 124o. [Beilstein 1 IV 3215.] LACHRYMATOR with toxic vapour.
Properties of Chloroacetone
| Melting point: | -44.5 °C |
| Boiling point: | 120 °C (lit.) |
| Density | 1.162 g/mL at 25 °C (lit.) |
| vapor pressure | 42 hPa (20 °C) |
| refractive index | n |
| Flash point: | 82 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Methanol (Slightly) |
| form | Liquid |
| color | Clear yellow to yellow-brown |
| Specific Gravity | 1.162 |
| PH | 4.3 (124g/l, H2O, 20°C) |
| explosive limit | 3.4%(V) |
| Water Solubility | 124 g/L (20 ºC) |
| Sensitive | Lachrymatory |
| Merck | 14,2114 |
| BRN | 605369 |
| Dielectric constant | 29.8(20℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. May discolour on exposure to light. STENCH. |
| CAS DataBase Reference | 78-95-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanone, 1-chloro-(78-95-5) |
| EPA Substance Registry System | Chloroacetone (78-95-5) |
Safety information for Chloroacetone
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloroacetone
Related products of tetrahydrofuran

You may like
-
 Chloroacetone, Stabilized CAS 78-95-5View Details
Chloroacetone, Stabilized CAS 78-95-5View Details
78-95-5 -
 Chloroacetone CAS 78-95-5View Details
Chloroacetone CAS 78-95-5View Details
78-95-5 -
 1-Chloro-2-propanone CAS 78-95-5View Details
1-Chloro-2-propanone CAS 78-95-5View Details
78-95-5 -
 Chloroacetone CAS 78-95-5View Details
Chloroacetone CAS 78-95-5View Details
78-95-5 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3