Heptafluorobutyric anhydride
Synonym(s):HFAA;HFBA;Perfluorobutyric anhydride
- CAS NO.:336-59-4
- Empirical Formula: C8F14O3
- Molecular Weight: 410.06
- MDL number: MFCD00000432
- EINECS: 206-410-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-30 18:41:17
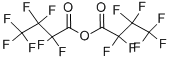
What is Heptafluorobutyric anhydride?
Chemical properties
clear colorless liquid
The Uses of Heptafluorobutyric anhydride
As a GC derivatizing agent, Heptafluorobutyric anhydride is used to prepare volatile derivatives of amino acids and other biologically active compounds.
The Uses of Heptafluorobutyric anhydride
Heptafluorobutyric anhydride (HFAA) is frequently used as an acylating agent for the derivatization of primary and secondary amines (Walle and Ehrsson, 1971). Commercially available Heptafluorobutyric anhydride may afford low yields of the derivative due to the presence of Heptafluorobutyric acid (Moodie et al., 1985). This contaminant may be removed by ditillation over phosphorus pentoxide (Walle and Ehrsson, 1970).
HFAA is used to increase volatility of an analyte, and to introduce fluorine atoms for better detection limits in gas chromatography applications.
suzuki reaction
The Uses of Heptafluorobutyric anhydride
Heptafluorobutyric anhydride is used as a derivatization reagent for gas chromatography - mass mass spectrometry analysis of the stereoisomer content of synthetic peptides. It is a GC derivatizing agent used to prepare volatile derivatives of amino acids and other biologically active compounds.
Definition
ChEBI: An acyclic carboxylic anhydride that is perfluorinated butyric anhydride. It is used as a derivatising reagent for gas chromatographic analyses.
General Description
Heptafluorobutyric anhydride is a perfluoroacylated acylation reagent, that forms stable, volatile derivatives with alcohols, amines, and phenols. It is majorly used to prepare electron-capturing derivatives for GC/electron capture detection. It is generally used with an acid scavenger, to help drive the reaction to completion and to prevent column damage from acidic by-products of the derivatization reaction. It is also used for the frequent testing of drugs of abuse such as, amphetamines and phencyclidine by GC-MS technique.
Properties of Heptafluorobutyric anhydride
| Melting point: | −43 °C(lit.) |
| Boiling point: | 108-110 °C(lit.) |
| Density | 1.674 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | None |
| storage temp. | room temp |
| solubility | Miscible with halogenated solvents. Immiscible with polar solvents. |
| form | Liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 1.653 |
| Water Solubility | REACTS |
| Sensitive | Hygroscopic |
| BRN | 856036 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 336-59-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Heptafluorobutyric anhydride(336-59-4) |
| EPA Substance Registry System | Heptafluorobutyric anhydride (336-59-4) |
Safety information for Heptafluorobutyric anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Heptafluorobutyric anhydride
| InChIKey | UFFSXJKVKBQEHC-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 336-59-4 Heptafluorobutyric anhydride 98%View Details
336-59-4 Heptafluorobutyric anhydride 98%View Details
336-59-4 -
 Heptafluorobutyric anhydride 95.00% CAS 336-59-4View Details
Heptafluorobutyric anhydride 95.00% CAS 336-59-4View Details
336-59-4 -
 Heptafluorobutyric Anhydride CAS 336-59-4View Details
Heptafluorobutyric Anhydride CAS 336-59-4View Details
336-59-4 -
 Heptafluorobutyric anhydride CAS 336-59-4View Details
Heptafluorobutyric anhydride CAS 336-59-4View Details
336-59-4 -
 Heptafluorobutyric anhydride CAS 336-59-4View Details
Heptafluorobutyric anhydride CAS 336-59-4View Details
336-59-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
