Butyric anhydride
Synonym(s):Butanoic anhydride;Butyric anhydride
- CAS NO.:106-31-0
- Empirical Formula: C8H14O3
- Molecular Weight: 158.2
- MDL number: MFCD00009389
- EINECS: 203-383-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
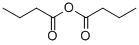
What is Butyric anhydride?
Description
Butyric anhydride, or Butanoic anhydride, is a chemical compound with the formula (CH3CH2CH2CO)2O.It is a colorless liquid that smells strongly of butyric acid, formed by its reaction with the moisture in the air.
Chemical properties
clear liquid
The Uses of Butyric anhydride
Butyric anhydride is used in the preparation of amidoamine dendron-based co-adsorbents, which finds application in dye- sensitized solar cells improvement. It is also used in the synthesis of butyrate ester, which is used as a perfume and flavor. Further, it acts as a fumigant to drive bees from their hives. In addition to this, it is used in food additives, textile auxiliaries, varnishes, perfumes, pharmaceuticals and disinfectants.
The Uses of Butyric anhydride
Manufacture of butyrates, drugs, and tanning agents.
The Uses of Butyric anhydride
Butyric anhydride can be used to prepare various flavor & fragrance compounds like neryl butyrate, geranyl butyrate, and butyl butyrllactate.
What are the applications of Application
Because of its odor, butyric anhydride has use as a fumigant to drive bees from their hives in products such as Bee - Go.
Preparation
To a cooled flask containing 88 gm (1.0 mole) of η-butyric acid at 10°C is added dropwise 28.0 gm (0.5 mole) of methoxyacetylene over a 1-hr period. After stirring the reaction mixture for 16 hr at 20°C the contents are distilled to afford the following three fractions: (1) b.p. 56°C, η& 1.3628, 23 gm (62%), methyl acetate; (2) b.p. 72-73°C (18 mm Hg), n% 1.3990, 10.0 gm (11%), butyric acid; (3) b.p. 91-92°C (18mmHg), 1.4118, 48.0 gm (61%), butyric anhydride.
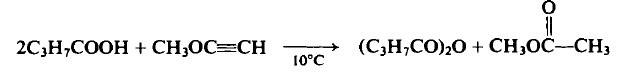
What are the applications of Application
Butyric anhydride is a chemical reagent used in the synthesis of amidoamine dendron-based co-adsorbents
General Description
Water-white liquid with an odor of rancid butter. Flash point 190°F. Density 8.0 lb / gal. Corrosive to metals and tissue. Low toxicity.
Air & Water Reactions
Slowly reacts with water to form butyric acid.
Reactivity Profile
Butyric anhydride reacts exothermically with water. The reaction is usually slow, but might become violent if local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Mildly toxic by ingestion. A corrosive liquid. When heated to decomposition it emits acrid smoke and irritating vapors.
Safety
Butyric anhydride is a combustible, corrosive liquid. It is considered water sensitive.
Purification Methods
Dry the anhydride by shaking it with P2O5, then distilling it. [Beilstein 2 IV 802.]
Properties of Butyric anhydride
| Melting point: | -75--66 °C (lit.) |
| Boiling point: | 198-199 °C (lit.) |
| Density | 0.967 g/mL at 25 °C (lit.) |
| vapor density | 5.45 (vs air) |
| vapor pressure | 10 mm Hg ( 79.5 °C) |
| refractive index | n |
| Flash point: | 190 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble (with decomposition)(lit.) |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | butter |
| explosive limit | 1.1%, 104°F |
| Water Solubility | Decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,1594 |
| BRN | 1099474 |
| Dielectric constant | 12.0(Ambient) |
| CAS DataBase Reference | 106-31-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, anhydride(106-31-0) |
| EPA Substance Registry System | Butyric anhydride (106-31-0) |
Safety information for Butyric anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Butyric anhydride
| InChIKey | YHASWHZGWUONAO-UHFFFAOYSA-N |
Butyric anhydride manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 n-Butyric anhydride CAS 106-31-0View Details
n-Butyric anhydride CAS 106-31-0View Details
106-31-0 -
 Butyric anhydride, GR 99%+ CAS 106-31-0View Details
Butyric anhydride, GR 99%+ CAS 106-31-0View Details
106-31-0 -
 Butyric Anhydride CASView Details
Butyric Anhydride CASView Details -
 Butyric anhydride 98% CAS 106-31-0View Details
Butyric anhydride 98% CAS 106-31-0View Details
106-31-0 -
 Butyric anhydride CAS 106-31-0View Details
Butyric anhydride CAS 106-31-0View Details
106-31-0 -
 Butyric Anhydride CAS 106-31-0View Details
Butyric Anhydride CAS 106-31-0View Details
106-31-0 -
 Butyric anhydride CAS 106-31-0View Details
Butyric anhydride CAS 106-31-0View Details
106-31-0 -
 Butyric anhydride CAS 106-31-0View Details
Butyric anhydride CAS 106-31-0View Details
106-31-0