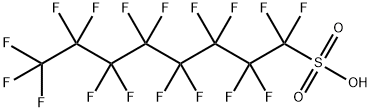
Perfluorooctanesulfonic acid
Synonym(s):Perfluorooctanesulfonic acid;PFOS
- CAS NO.:1763-23-1
- Empirical Formula: C8HF17O3S
- Molecular Weight: 500.13
- MDL number: MFCD00042454
- EINECS: 217-179-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
What is Perfluorooctanesulfonic acid?
Description
Perfluorooctanesulfonic acid, usually abbreviated to PFOS, was once a widely used surfactant in fabric protectors, firefighting foams, and photolithographic chemical mixtures. It was introduced in 1949 by 3M Co. (then known as Minnesota Mining and Manufacturing Co.).
By 1968, traces of PFOS began to appear in human blood. 3M began to phase it out in 2000, but it and similar perfluorinated compounds continue to be produced in China.
Because of the multiple hazards imposed by PFOS and its cousins (see hazard information box), efforts have been made to develop lower fluorine content or fluorine-free foams for several years. Specifically, perfluorinated C6 surfactants have lower toxicological and environmental profiles than the C8s. Some of the C6s meet US military foam standards.
Thus far, the C6 products have significantly outperformed fluorine-free surfactants. But the pressure is on to develop better non-fluorine foams because last year Congress passed, and the president signed, an act that allows civilian airports to use these foams to fight fires.
Description
Perfluorooctanesulfonic acid or perfluorooctane sulfonate (PFOS), is a man-made fluorosurfactant and global pollutant. It is a very useful catalyst in chemical industry. It can catalyze the Intramolecular Hydroamination of Olefinic Sulfonamides in Fluorous Biphase System (FBS)1. It can also catalyze ring-opening reactions of methylenecyclopropanes with aromatic amines, sulfonamides and alcohols in supercritical carbon dioxide2. It is useful in the nitroaldol reaction in fluorous media, which is an important improvement of the chemo-selective addition of aldehyde3.
Chemical properties
Perfluorooctane sulfonate can exist as a liquid or a powder. It is soluble in water (NTP, 2013). Perfluorooctane sulfonate has the ability to react with strong oxidizing agents. Upon decomposition, PFOS can form carbon oxides, sulfur oxides, and hydrogen fluoride. Additional information related to the physical and chemical properties of PFOS are not currently available.
The Uses of Perfluorooctanesulfonic acid
Heptadecafluorooctanesulfonic acid may be used as an analytical reference standard for the determination of the analyte in water samples using solid-phase microextraction combined with high-performance liquid chromatography with electrospray-ion trap mass spectrometry.
The Uses of Perfluorooctanesulfonic acid
Perfluorooctane sulfonate (PFOS, 1-octanesulfonic acid, heptadecafluorooctanesulfonic acid, perfluorooctanesulfonic acid, 1-perfluorooctanesulfonic acid) is an eight-carbon compound in the perfluoroalkyl family of chemicals. Perfluorooctane sulfonate is used in a variety of applications, including textiles, leather products, cleaning products, and pesticides. Its main use was as a stain repellent on carpet, furniture, and other consumer products. 3M was the primary manufacturer of the chemical until it voluntarily phased it out in 2002 (USEPA, 2012). Perfluorooctane sulfonate is not manufactured any longer in the United States, but it can be imported for use in specific applications.
Definition
ChEBI: Perfluorooctane-1-sulfonic acid is a perfluoroalkanesulfonic acid that is octane-1-sulfonic acid in which all seventeen of the hydrogens that are attached to carbons hvae been replaced by fluorines. It has a role as an antilipemic drug and a persistent organic pollutant. It is functionally related to an octane-1-sulfonic acid.
General Description
Heptadecafluorooctanesulfonic acid belongs to the class of perfluorinated alkyl acids and is emerging as persistent organic contaminants in the environment.
Health effects
Exposure to unsafe levels of PFOA/PFOS concentrations through drinking water may result in health effects including developmental effects to fetuses during pregnancy, cancer, liver effects, immune effects and thyroid effects.
Environmental considerations
Perfluorooctane sulfonic acid (PFOS) is one of a group of related chemicals known as perfluorinated alkylated substances (PFAS). These are also called perfluorochemicals (PFCs). This group of chemicals is commonly used in a wide range of industrial processes and is found in many consumer products. PFOS can enter groundwater from multiple sources, including sewage treatment plants, industrial sites, landfills, and places where it is used in firefighting foam such as airports, firefighter training sites, and military installations. Fish and shellfish can take up PFOS from water contaminated with the chemical.
References
[1] YAN YIN Gang Z. Heptadecafluorooctanesulfonic acid (C8F17SO3H) catalyzed intramolecular hydroamination of olefinic sulfonamides in fluorous biphase system (FBS)[J]. Journal of Fluorine Chemistry, 2007, 128 1: Pages 40-45. DOI:10.1016/j.jfluchem.2006.09.012.
[1] SHI M, CHEN Y, XU B, et al. Heptadecafluorooctanesulfonic acid catalyzed ring opening reactions of methylenecyclopropanes with aromatic amines, sulfonamides and alcohols in supercritical carbon dioxide[J]. Green Chemistry, 2003, 1: 85-88. DOI:10.1039/B210988C.
https://www.sciencedirect.com/science/article/pii/S1566736707001240
https://www.p65warnings.ca.gov/fact-sheets/pfos-perfluorooctane-sulfonate-or-perfluorooctane-sulfonic-acid
https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf
https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/pfosinfo.pdf
[1] PING QI. Aquatic predicted no-effect-concentration derivation for perfluorooctane sulfonic acid[J]. Environmental Toxicology and Chemistry, 2011, 30 4: 836-842. DOI:10.1002/etc.460.
Properties of Perfluorooctanesulfonic acid
| Melting point: | 90 °C |
| Boiling point: | 260 °C |
| Density | 1.25 |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | 680 mg/l |
| solubility | Ethanol: 10 mg/ml |
| appearance | white powder |
| pka | -3.27±0.50(Predicted) |
| BRN | 1813859 |
| CAS DataBase Reference | 1763-23-1(CAS DataBase Reference) |
| EPA Substance Registry System | Perfluorooctane sulfonic acid (1763-23-1) |
Safety information for Perfluorooctanesulfonic acid
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation H372:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Perfluorooctanesulfonic acid
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1