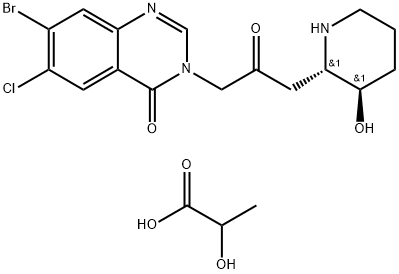
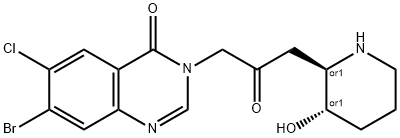
Halofuginone Lactate
- CAS NO.:82186-71-8
- Empirical Formula: C19H23BrClN3O6
- Molecular Weight: 504.76
- MDL number: MFCD00144429
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
What is Halofuginone Lactate?
Description
Halofuginone Lactate is a halogenated derivative of febrifugine, a natural quinazolinone-containing compound found in the Chinese herb D. febrifuga.
The Uses of Halofuginone Lactate
Halofuginone is a halogenated derivative of Febrifugine (F228500). Halofuginone is used as an antiprotozoal. It is a coccidiostat used in veterinary medicine. It is a synthetic halogenated derivative of febrifugine, a natural quinazolinone alkaloid which can be found in the Chinese herb Dichroa febrifuga (Chang Shan).Collgard Biopharmaceuticals is developing halofuginone for the treatment of scleroderma and it has received orphan drug designation from the U.S. Food and Drug Administration.
Integration of halofuginone lactate treatment and disinfection with p-chloro-m-cresol to control natural cryptosporidiosis in calves
What are the applications of Application
Halofuginone lactate is a synthetic product of the quinazolinone group with antiprotozoal activity, especially against Coccidia and Theileria species. It is effective in many cases of calf cryptosporidiosis, as has been shown in studies in Europe and Canada. Also, halofuginone is effective in lamb cryptosporidiosis. Halofuginone prevented natural infections in kids in France and in Greece[1].
Pharmacokinetics
Halofuginone lactate is a salt whose antiprotozoal properties and efficacy against Cryptosporidium parvum have been demonstrated both in in vitro conditions and artificial and natural infections. The compound has a cryptosporidiostatic effect on Cryptosporidium parvum. It is mainly active in the free stages of the parasite (sporozo?te, merozo?te). The concentrations to inhibit 50% and 90% of the parasites in an in vitro test system are IC50 < 0.1 μg/ml and IC90 of 4.5 μg/ml, respectively. The bioavailability of the drug in the calf following a single oral administration is about 80%. The time necessary to obtain the maximum concentration Tmax is 11 hours. The maximum concentration in plasma Cmax is 4 ng/ml. The apparent volume of distribution is 10 l/kg. The plasmatic concentrations of halofuginone after repeated oral administrations are comparable to the pharmacokinetic pattern after a single oral treatment. Unchanged halofuginone is the major component in the tissues. The highest values have been found in the liver and the kidney. The product is mainly excreted in the urine. The terminal elimination half-life is 11.7 hours after IV administration and 30.84 hours after single oral administration.
References
[1] N.D. Giadinis . “Efficacy of halofuginone lactate for the treatment and prevention of cryptosporidiosis in goat kids: An extensive field trial.” Small Ruminant Research 76 3 (2008): Pages 195-200.
Properties of Halofuginone Lactate
| storage temp. | 0-6°C |
Safety information for Halofuginone Lactate
Computed Descriptors for Halofuginone Lactate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1