Halofuginone
- CAS NO.:55837-20-2
- Empirical Formula: C16H17BrClN3O3
- Molecular Weight: 414.68
- MDL number: MFCD09834143
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
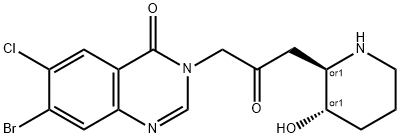
What is Halofuginone?
Description
Halofuginone hydrobromide (Halofuginone) is a specific collagen Type I inhibitor that antagonize or inhibit the development of new blood vessels, hence can prevent intimal hyperplasia at a vascular anastomosis. It is used in the treatment or prevention of coccidiosis in both humans and animals.
Chemical properties
Off-White Solid
The Uses of Halofuginone
Halofuginone is a halogenated derivative of febrigugine. It is Used as an antiprotozoal.
The Uses of Halofuginone
Halofuginone is a coccidiostat for young chickens and young turkeys. In higher doses, halofuginone is a growth depressant, impairs feed utilization, and reduces feed intake. In rats it causes alopecia. The compound is prohibited from use during the last four days before slaughter (withdrawal period).
Halofuginone is extracted from enzyme-digested chicken liver as a free base into ethyl acetate and partitioned into ammonium acetate buffer solution. The halofuginone is concentrated with Sep-Pak C18 cartridges and eluted with methyl alcohol. The eluant is evaporated to dryness and the residue dissolved in the HPLC mobile phase. Analysis is achieved by HPLC using a BONDAPAK C18 column and a variable wavelength UV detector.
The Uses of Halofuginone
Halofuginone is a halogenated derivative of Febrifugine (F228500). Halofuginone is used as an antiprotozoal.
brand name
Stenorol(Roussel-UCLAF, France).
Biological Activity
Halofuginone is a halogenated derivative of febrifugine, a natural quinazolinone-containing compound found in the Chinese herb D. febrifuga. It has antimalarial and anticoccidial actions. In mammals, halofuginone at 10 ng/ml down-regulates Smad3, blocking TGF-β signaling and preventing both the differentiation of fibroblasts to myofibroblasts and the transitioning of epithelial cells to mesenchymal cells. Through this action, halofuginone blocks fibrosis and tumor progression in a variety of different models. This compound also competitively inhibits prolyl-tRNA synthetase (Ki = 18.3 nM), activating the amino acid starvation response. This prevents the differentiation of TH17 cells, blunting an autoimmune response.
Synthesis
A scalable total synthesis of halofuginone has been accomplished. This synthetic route features a total of 12 steps of highly efficient reactions, without any chromatographic purification. Halofuginone was obtained in 17% overall yield and over 98.5% HPLC purity. All the reaction conditions are mild and reliable. In addition, no hazardous materials are used or produced. All reagents are commercially available and inexpensive. This route is safe, robust, scalable, cost-effective, and environmentally benign.
A Scalable Total Synthesis of Halofuginone
Mode of action
Halofuginone is an analog of febrifugine-an alkaloid originally isolated from the plant Dichroa febrifuga. During recent years, halofuginone has attracted much attention because of its wide range of beneficial biological activities, which encompass malaria, cancer, and fibrosis-related and autoimmune diseases. At present two modes of halofuginone actions have been described:
(1) Inhibition of Smad3 phosphorylation downstream of the TGFβ signaling pathway results in inhibition of fibroblasts-to-myofibroblasts transition and fibrosis.
(2) Inhibition of prolyl-tRNA synthetase (ProRS) activity in the blood stage of malaria and inhibition of Th17 cell differentiation thereby inhibiting inflammation and the autoimmune reaction by activation of the amino acid starvation and integrated stress responses.
Properties of Halofuginone
| Melting point: | >150°C dec. |
| Boiling point: | 595.8±60.0 °C(Predicted) |
| Density | 1.73±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO : 9 mg/mL (21.70 mM) |
| pka | 14.61±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 55837-20-2(CAS DataBase Reference) |
Safety information for Halofuginone
Computed Descriptors for Halofuginone
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran




You may like
-
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
![2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
740842-50-6 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3