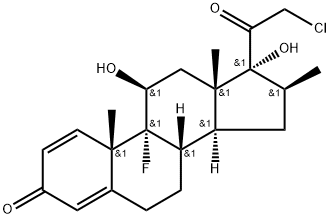
HALOBETASOL
- CAS NO.:98651-66-2
- Empirical Formula: C22H27ClF2O4
- Molecular Weight: 428.9
- MDL number: MFCD00868926
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
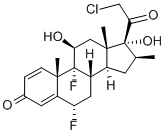
What is HALOBETASOL?
Absorption
Ulobetasol lotion reaches a Cmax of 201.1 ± 157.5 pg/mL, with a Tmax of 3 hours, and an AUC of 1632 ± 1147 pg*h/mL. Absorption can be influenced by skin integrity, the vehicle used, inflammation, or disease processes.
Toxicity
Data regarding acute overdoses of glucocorticoids are rare. Chronic high doses of glucocorticoids can lead to the development of cataract, glaucoma, hypertension, water retention, hyperlipidemia, peptic ulcer, pancreatitis, myopathy, osteoporosis, mood changes, psychosis, dermal atrophy, allergy, acne, hypertrichosis, immune suppression, decreased resistance to infection, moon face, hyperglycemia, hypocalcemia, hypophosphatemia, metabolic acidosis, growth suppression, and secondary adrenal insufficiency. Overdose may be treated by adjusting the dose or stopping the corticosteroid as well as initiating symptomatic and supportive treatment.
The Uses of HALOBETASOL
Halobetasol is a corticosteroid, an anti-inflammatory drug that is used to treat various dermatological conditions which mainly consist of various forms of psoriasis.
Background
Ulobetasol is a highly potent corticosteroid. It is structurally related to clobetasol. Due to its high potency, it is mainly prescribed in the treatment of severe plaque psoriasis and corticosteroid responsive dermatoses.
Ulobetasol was granted FDA approval on 17 December 1990.
Indications
Ulobetasol cream and ointment are indicated in the treatment of inflammatory and pruritic corticosteroid responsive dermatoses. Ulobetasol lotion is indicated in the treatment of plaque psoriasis.
brand name
Ultravate (Westwood-Squibb).
Pharmacokinetics
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Ulobetasol has a moderate duration of action as it is applied once or twice daily. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Metabolism
Not Available
Properties of HALOBETASOL
| Melting point: | >218°C (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Pale Yellow |
| Stability: | Hygroscopic |
Safety information for HALOBETASOL
Computed Descriptors for HALOBETASOL
Abamectin manufacturer
Varanous Labs Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 98651-66-2 Halobetasol Propiote 98%View Details
98651-66-2 Halobetasol Propiote 98%View Details
98651-66-2 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4