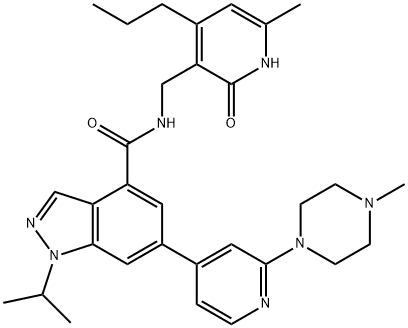
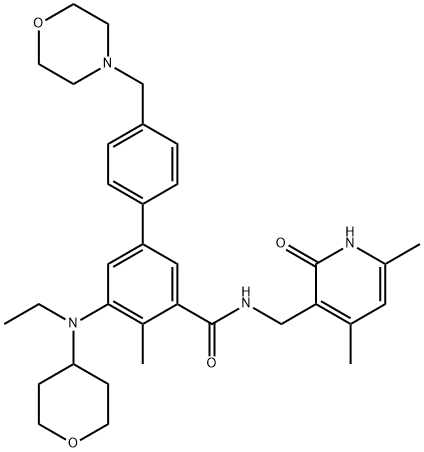
GSK 343
Synonym(s):N-[(1,2-Dihydro-6-methyl-2-oxo-4-propyl-3-pyridinyl)methyl]-1-(1-methylethyl)-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl]-1H-indazole-4-carboxamide;N-[(6-Methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl]-6-[2-(4-methylpiperazin-1-yl)pyridin-4-yl]-1-(propan-2-yl)-1H-indazole-4-carboxamide
- CAS NO.:1346704-33-3
- Empirical Formula: C31H39N7O2
- Molecular Weight: 541.69
- MDL number: MFCD25563250
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is GSK 343?
The Uses of GSK 343
GSK343 has been used:
- as an inhibitor of?enhancer of zeste homolog 2 (EZH2) to assess the impact of chromatin condensation on?cell migration
- as histone methyl transferase inhibitor, to suppress the Polyandrocarpa misakiensis, mitochondrial non-coding-region (NCR) PmNCR?reporter gene expression, facilitated?by TC14-3
- as a supplement in the growth medium to inhibit the generation of H3K27me3 or to inhibit RNA polymerase II
- as EZH2 inhibitor to treat castration-resistant neuroendocrine prostate cancer (CRPC-NE)
What are the applications of Application
GSK343 is an inhibitor of the histone lysine methyltransferase ENX-1
Definition
ChEBI: GSK343 is a member of the class of indazoles that is 1-isopropyl-1H-indazole-4-carboxamide in which the nitrogen of the carboxamide group is substituted by a (6-methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl group and in which the indazole ring is substituted at position 6 by a 2-(4-methylpiperazin-1-yl)pyridin-4-yl group. A highly potent and selective EZH2 inhibitor (IC50 = 4 nM). It has a role as an EC 2.1.1.43 (enhancer of zeste homolog 2) inhibitor, an apoptosis inducer and an antineoplastic agent. It is a N-alkylpiperazine, a secondary carboxamide, an aminopyridine, a pyridone, a N-arylpiperazine and a member of indazoles.
Biological Activity
gsk343 is a selective and sam-competitive inhibitor of the histone lysine methyltransferase ezh2 with ic50 value of 4 nm [1].ezh2 (enhancer of zestehomolog 2) is a catalytic subunit of the protein complex (polycomb repressive complex 2, prc2). it catalyzes the methylation of h3k27 through transferring the methyl group of sam to h3k27. the trimethylation of h3k27 subsequently results in the transcription suppression of target genes such as runx3, foxc1 and brca1. the overexpression and mutation of ezh2 have been found in many sorts of tumors demonstrating that ezh2 is an attractive target for the treatment of cancers. as a potent ezh2 inhibitor, gsk343 inhibits the activity of the enzyme via competing with the cofactor sam [1, 2].gsk343 is a selective ezh2 inhibitor. it showed no significant inhibitory effects on other enzymes requiring sam as cofactor, including dnmt, mll, prmt and setmar. gsk343 exerted a certain degree of effective on ezh1 with ic50 value of 240 nm since ezh1 was quite homologous to ezh2. in cultured hcc1806 breast cancer cells, treatment of gsk343 dose-dependently reduced h3k27me3 with ic50 value of 174 nm. besides that, gsk343 was found to inhibit cell proliferation in some breast and prostate cancer cells. in lncap cells, gsk343 suppressed cell growth with ic50 value of 2.9 μm. in human eoc cells, gsk343 notably inhibited cell invasion and induced cell apoptosis. gsk343 was also found to induce lc3-ii accumulation and autophagy in a549, mda-mb-231 and hepg2 cell. it enhanced the antitumor activity of sorafenib which was a multikinase inhibitor and could decrease the expression of ezh2 in hepg2 cells [1, 3 and 4].gsk343 is only used as a tool to investigate ezh2 in vitro because of its high clearance in the animal model [1].
Biochem/physiol Actions
GSK343 is a potent, specific inhibitor of the histone H3-lysine 27 (H3K27) methyltransferase EZH2. GSK343 inhibits EZH2 enzymatic activity with an IC50 of 4 nM. The compound displays 60 fold selectivity for EZH2 vs. EZH1, and 1000 fold or greater selectivity against other histone methyltransferases. The IC50 for inhibition of H3K27 methylation is < 200 nM in HCC1806 cells. For full characterization details, please visit the GSK343 probe summary on the Structural Genomics Consortium (SGC) website.To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
storage
Store at -20°C
References
[1] verma s k, tian x, lafrance l v, et al. identification of potent, selective, cell-active inhibitors of the histone lysine methyltransferase ezh2. acs medicinal chemistry letters, 2012, 3(12): 1091-1096.
[2] ferraro a, boni t, pintzas a. ezh2 regulates cofilin activity and colon cancer cell migration by targeting itga2 gene. plos one, 2014, 9(12): e115276.
[3] amatangelo m, garipov a, li h, et al. three-dimensional culture sensitizes epithelial ovarian cancer cells to ezh2 methyltransferase inhibition. cell cycle, 2013, 12(13): 2113-2119.
[4] liu t p, lo h l, wei l s, et al. s-adenosyl-l-methionine-competitive inhibitors of the histone methyltransferase ezh2 induce autophagy and enhance drug sensitivity in cancer cells. anti-cancer drugs, 2015, 26(2): 139.
Properties of GSK 343
| Boiling point: | 797.4±60.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| pka | 11.84±0.10(Predicted) |
| color | , white to beige to brown |
Safety information for GSK 343
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for GSK 343
| InChIKey | ULNXAWLQFZMIHX-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![3-Cyclopentene-1,2-diol, 5-(4-aMino-1H-iMidazo[4,5-c]pyridin-1-yl)-3-(hydroxyMethyl)-, (Hydrochloride) (1:1), (1S,2R,5R)-](https://img.chemicalbook.in/CAS/GIF/120964-45-6.gif)

![6-[[3-[(Dimethylamino)carbonyl]phenyl]sulfonyl]-4-[(3-methoxyphenyl)amino]-8-methyl-3-quinolinecarboxamide](https://img.chemicalbook.in/CAS2/GIF/801312-28-7.gif)
You may like
-
 GSK343 >95% CAS 1346704-33-3View Details
GSK343 >95% CAS 1346704-33-3View Details
1346704-33-3 -
 GSK343 CAS 1346704-33-3View Details
GSK343 CAS 1346704-33-3View Details
1346704-33-3 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
