GS-441524
- CAS NO.:1191237-69-0
- Empirical Formula: C12H13N5O4
- Molecular Weight: 291.26
- MDL number: MFCD32666994
- EINECS: 200-001-8
- Update Date: 2024-11-19 23:02:33
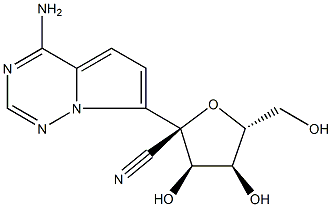
What is GS-441524?
Description
GS-441524 is the active prodrug of the broad-spectrum antiviral Remdesivir. GS-441524 is an adenosine nucleotide analog in viral RNA production. It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production (RNA synthesis arrest), either by terminating RNA chains or causing mutations. It inhibits MERS-CoV or SARS-CoV-infected HAE cultures (EC50=74nM and 69nM) and murine hepatitis virus (MHV) (EC50=30nM). In vivo, remdesivir (25 and 50 mg/kg) reduces lung viral titers and prevents weight loss in a mouse model of SARS-CoV infection. Remdesivir is used as a treatment for Ebola virus disease and Marburg virus infections and other single stranded RNA viruses such as respiratory syncytial virus, Junin virus, Lassa fever virus, Nipah virus, Hendra virus and the coronaviruses (including MERS and SARS viruses). Remdesivir is effective in the control of 2019-nCoV (COVID-19) infection in vitro. It now also has been studied as potential treatment of SARS-CoV-2 infections.
The Uses of GS-441524
GS-441524 is originally identified as an active metabolite of Remdesivir that is an antiviral drug, a novel nucleotide analog prodrug.
Definition
ChEBI: GS-441524 is a C-nucleoside analog that is (2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile substituted by a 4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl group at position 2. It is the active metabolite of remdesivir and exhibits a broad range of inhibitory activity against various RNA viruses including HCV, parainfluenza and SARS-CoV. It has a role as a drug metabolite, an antiviral agent and an anticoronaviral agent. It is a pyrrolotriazine, a nitrile, a C-nucleoside and an aromatic amine.
Acquired resistance
Resistance to GS-441524 can exist at the time of diagnosis, but this is uncommon. Rather, it tends to occur during treatment and is often partial at first and necessitates a higher dosage to accommodate for it. It can become total in some cats. Resistance is the biggest problem in cats with neurological disease, especially those that present with neurological disease or develop brain infections during treatment or during a relapse after what appears to have been a successful treatment. Many cats with partial drug resistance can be treated for their disease signs but will relapse as soon as the treatment is stopped. There have been cats "treated" for FIP for over a year with no cure, but ultimately resistance becomes worse or the owner runs out of money.
Biological Activity
GS 441524 is a viral RNA-dependent RNA polymerase (RdRp) inhibitor and broad spectrum antiviral nucleotide; active metabolite of Remdesivir. Competes with natural nucleoside triphosphates, blocking viral RNA synthesis. Exhibits antiviral activity against viruses from Coronaviridae family including SARS-CoV-2 in vitro, and SARS-CoV and feline infectious peritonitis virus (FIPV) in vitro and in vivo. Inhibits SARS-CoV and MERS-CoV- infected HAE cultures (EC50 values are 0.18 and 0.86 μM, respectively) and inhibits murine hepatitis virus (MHV) (EC50 = 1.1 μM).
Pharmacokinetics
GS-441524 is a nucleoside analogue that terminates the RNA chain of viral RNA-dependent RNA polymerase. It was developed by Gilead Sciences, Inc as part of their research into human RNA virus disease such as Ebola. GS-441524 is a small molecule that exhibits potent antiviral activity against a number of RNA viruses, including the zoonotic severe acute respiratory syndrome (SARS) coronavirus ( Cho et al., 2012). GS-5734 is a phosphoramidate prodrug of GS-441524 that has been previously shown to inhibit the replication of several taxonomically diverse RNA viruses such as Middle East respiratory syndrome virus, Ebola virus, Lassa fever virus, Junin virus and respiratory syncytial virus, while having low cytotoxicity in a wide-range of cell lines ( Sheahan et al., 2017 ). GS-5734 has also been shown to protect rhesus monkeys from experimental Ebola virus infection ( Warren et al., 2016 ).
Side Effects
GS-441524 treatment is amazingly free of systemic side effects. It can cause minor kidney damage in some cats, but this does not progress to overt renal disease. Systemic drug reactions of the vasculitis type have been seen in a few cats and can be confused with injection site reactions. However, these drug reactions are at non-injection sites and are often self-limiting or respond well to a short-term low dose of steroids. The major side-effect of GS treatment is pain at the injection sites, which varies from cat to cat and according to the injection prowess of the person doing the treatment (usually the owner). Injection site sores are a problem with some owners and usually occur when the injection site is not moved around the body (stay away from between the shoulders) and not given into the muscle and nerve layers below the subcutis. I recommend selecting sites starting an inch behind the shoulder blades, down the back to 1-2 inches before the tailhead, and one third to one-half of the way down the chest and abdomen. Many people use gabapentin before injections to help ease the pain. Injection site sores are cleared of surrounding hair and gently cleaned 4 or more times a day with sterile cotton balls soaked in 1:5 dilution of household hydrogen peroxide. They usually do not require any more sophisticated treatment and heal within 2 weeks or so.
storage
Store at -20°C
Preparation and handling
GS-441524 is supplied as a solid. A stock solution may be made by dissolving the GS-441524 in the solvent of choice, which should be purged with an inert gas. It is soluble in organic solvents such as DMSO and dimethyl formamide. The solubility of GS-441524 in these solvents is approximately 10 mg/ml.
GS-441524 is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, It should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. It has a solubility of approximately 0.16 mg/ml in a 1:5 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day.
Mode of action
GS-441524 undergoes intracellular phosphorylation by cellular kinases, first to a nucleoside monophosphate and then to an active triphosphate metabolite (NTP)( Cho et al., 2012; Sheahan et al., 2017; Warren et al., 2016). This active NTP analog competes with natural nucleoside triphosphates to inhibit viral RNA synthesis. Specifically, the active form of GS-441524 inhibits RNA-dependent RNA polymerase-mediated transcription by terminating nascent viral transcript prematurely. We hypothesized that, once activated in feline cells, GS-441524 would attenuate FIPV replication with minimal cytotoxicity in vitro and effectively treat cats with experimentally induced FIP.
References
1) Remdesivir and GS-441524 Retain Antiviral Activity against Delta, Omicron, and Other Emergent SARS-CoV-2 Variants DOI:10.1128/aac.00222-22
2) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys: T.K. Warren, et al.; Nature 531, 381 (2016) DOI:10.1038/nature17180
3) Late Ebola virus relapse causing meningoencephalitis: a case report: M. Jacobs, et al.; Lancet 388, 498 (2016) DOI:10.1016/S0140-6736(16)30386-5
4) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses: T.P. Sheahan, et al.; Sci. Transl. Med. 9, eaal3653 (2017) DOI:10.1126/scitranslmed.aal3653
5) Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4- amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses; D. Siegel, et al.; J. Med. Chem. 60, 1648 (2017) DOI:10.1021/acs.jmedchem.6b01594
6) Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease: M.L. Agostini, et al.; mBio 9, e00221 (2018) DOI: 10.1128/mBio.00221-18
7) Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir: E.P. Tchesnokov, et al.; Viruses 11, E326 (2019) DOI:10.3390/v11040326
8) Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge: M.K. Lo, et al.; Sci. Transl. Med. 11, eaau9242 (2019) DOI:10.1126/scitranslmed.aau9242
9) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase: A.J. Brown, et al.; Antiviral Res. 169, 104541 (2019) DOI:10.1016/j.antiviral.2019.104541
10) Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection: R. de Wit, et al.; PNAS (Epub ahead of print) (2020) DOI:10.1073/pnas.1922083117
11) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro: M. Wang, et al.; Cell Res. 30, 269 (2020) DOI:10.1038/s41422-020-0282-0
12) The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies DOI: 10.1016/j.vetmic.2018.04.026
Properties of GS-441524
| Melting point: | > 233° C (dec.) |
| Density | 1.84±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (>25 mg/ml) |
| form | solid |
| pka | 12.13±0.70(Predicted) |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChI | InChI=1/C12H13N5O4/c13-4-12(10(20)9(19)7(3-18)21-12)8-2-1-6-11(14)15-5-16-17(6)8/h1-2,5,7,9-10,18-20H,3H2,(H2,14,15,16)/t7-,9-,10-,12+/s3 |
| CAS DataBase Reference | 1191237-69-0 |
Safety information for GS-441524
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for GS-441524
| InChIKey | BRDWIEOJOWJCLU-QHRJDZJMNA-N |
| SMILES | C([C@]1(C2=CC=C3C(=NC=NN23)N)[C@@H]([C@H](O)[C@@H](CO)O1)O)#N |&1:1,12,13,15,r| |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
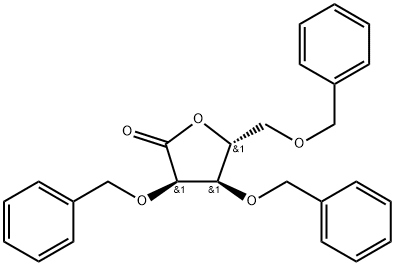
![(2R,3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol](https://img.chemicalbook.in/CAS/20210111/GIF/1911578-73-8.gif)
![4-amino-7-iodopyrrolo[2,1-f][1,2,4]triazine](https://img.chemicalbook.in/CAS/20200611/GIF/1770840-43-1.gif)



You may like
-
![1191237-69-0 (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/670a89dc-a6f3-4e61-9ef2-0169ddacc086.png) 1191237-69-0 (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile 98%View Details
1191237-69-0 (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile 98%View Details
1191237-69-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1