Germanium oxide
Synonym(s):Germanium dioxide;Germanium(IV) oxide
- CAS NO.:1310-53-8
- Empirical Formula: GeO2
- Molecular Weight: 104.64
- MDL number: MFCD00011030
- EINECS: 215-180-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:58
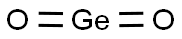
What is Germanium oxide?
Description
Germanium oxide also called Germanium dioxide is the oxide of germanium, an inorganic compound, featuring the chemical formula GeO2. It is formed as a passivation layer on pure germanium after exposure to oxygen. Germanium dioxide generally has a low toxicity, but shows severe nephrotoxicity at higher doses. Germanium dioxide is still offered on the market in some questionable miracle therapies. Exposure to high doses of germanium dioxide can lead to germanium poisoning.
Chemical properties
White powder, hexagonal, tetragonal,
and amorphous.
The Uses of Germanium oxide
Germanium oxide can be used in Phosphors, transistors and diodes, infraredtransmitting glass.
The Uses of Germanium oxide
When Germanium dioxide is 99.999% pure, it is used as a semiconductor in transistors, in diodes, and to make special infrared transmitting glass.
The Uses of Germanium oxide
Germanium dioxide is used as an optical material for wide-angle lenses and in optical microscope objective lenses. It is used as a catalyst in the preparation of polyethylene terephthalate resin. Further, it is used as a feedstock for the manufacturing of phosphors and semiconductor materials. It is also used in transistors and diodes, and infrared transmitting glass.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
The oxide (GeO2) is usually prepared by hydrolysing redistilled GeCl4 and igniting it in order to remove H2O and chloride. It can be further purified by dissolving in hot H2O (solubility is 4g/L cold) evaporating and drying the residual crystalline solid. When the soluble form (which is produced in H2O at 355o) is heated for 100hours, it is converted to the insoluble form. This form is stable at temperatures up to 1033o, and fusion at 1080o for 4hours causes complete de-vitrification and it reverts to the soluble form. [Müller & Blank J Am Chem Soc 46 2358 1924, Dennis & Laubengayer J Am Chem Soc 47 1945 1925, Laubengayer & Morton J Am Chem Soc 54 2303 1932, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 706 1963.]
Structure and conformation
Germanium oxide ccurs in two crystalline and one amorphous modifications: (1) a tetragonal rutile form, refractive index 2.05, density 6.24 g/cm3 at 20°C. (2) white hexagonal quartz modification, refractive index 1.735, density 4.70 g/cm3 at 18°C, and (3) a glassy amorphous form, refractive index 1.607, density 3.64 g/cm3 at 20°C. The tetragonal form is practically insoluble in water, while the hexagonal and the amorphous modifications have low solubilities; 0.45 and 0.52% respectively, at 25°C. Aqueous solutions are acidic due to formation of metagermanic acid, H2GeO3. Hexagonal modification converts to a tetragonal crystal system when heated at 350°C in water under pressure. Both crystalline forms convert to a glass-like amorphous GeO2 when heated at 1,100°C.
Properties of Germanium oxide
| Melting point: | >400 °C(lit.) |
| Density | 6.239 g/mL at 25 °C |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.99 |
| storage temp. | no restrictions. |
| solubility | ethylene glycol: soluble |
| form | Powder/Flake/Crystalline/Beads/Lump, Etc. |
| color | White |
| Specific Gravity | 6.239 |
| Water Solubility | 0.4 g/100 mL |
| Merck | 14,4409 |
| Solubility Product Constant (Ksp) | pKsp: 57 |
| CAS DataBase Reference | 1310-53-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Germanium dioxide(1310-53-8) |
| EPA Substance Registry System | Germanium oxide (GeO2) (1310-53-8) |
Safety information for Germanium oxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Germanium oxide
| InChIKey | YBMRDBCBODYGJE-UHFFFAOYSA-N |
Germanium oxide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8 -
 Germanium(IV) oxide CAS 1310-53-8View Details
Germanium(IV) oxide CAS 1310-53-8View Details
1310-53-8
