Furfuryl alcohol
Synonym(s):2-(Hydroxymethyl)furan;2-Furylmethanol;Furfuryl alcohol
- CAS NO.:98-00-0
- Empirical Formula: C5H6O2
- Molecular Weight: 98.1
- MDL number: MFCD00003252
- EINECS: 202-626-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
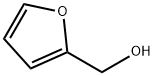
What is Furfuryl alcohol?
Description
Furfural alcohol is a colorless to amber liquidthat darkens on exposure to light. It has a faint, burningodor. Molecular weight=98.11; Specific gravityFurfuryl alcohol 1371(H2O:1)=1.13; Boiling point: 171℃; Melting point:215℃; Vapor pressure=6 mmHg at 25℃; Flash point =65℃ (cc); 75℃ (oc); Autoignition temperature=491℃.Explosive limits: LEL=1.8%; UEL=16.3%. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 2, Reactivity 1. Soluble in water.
Chemical properties
clear yellow liquid
Chemical properties
Furfural alcohol is a colorless to amber liquid that darkens on exposure to light. It has a faint, burning odor.
Chemical properties
Furfuryl alcohol has a very mild, warm, oily, “burnt” odor and a cooked sugar taste.
Physical properties
Clear, colorless to pale yellow liquid with an irritating odor. Darkens to yellowish-brown on exposure to air. A detection odor threshold concentration of 32 mg/m3 (8.0 ppmv) was determined by Jacobson et al. (1958).
Occurrence
Reported found in roasted almonds, cooked apple, apple juice, roasted barley, beans, beef fat, canned beef stew, beer, brandy, white bread, cocoa, cocoa bean, roasted coffee, roasted flberts, honey, heated skim milk, dried mushrooms, roasted onion, yellow passion fruit, roasted peanuts, pineapple, popcorn, potato chips, roasted sesame seeds, cheeses, milk, meats, grape wines, cognac, whiskies, soybean products, coconut, corn oil, shrimps, clams and other sources
The Uses of Furfuryl alcohol
Furfuryl Alcohol has been obtained by yeast reduction of furfural. Furfuryl Alcohol is used as solvent and in the manufacturing of wetting agents, resins.
The Uses of Furfuryl alcohol
Solvent; manufacture of wetting agents, resins.
The Uses of Furfuryl alcohol
Colorless liquid that turns dark in air
What are the applications of Application
Furfuryl alcohol is used in the synthesis of continuous titanium carbide nanofibers/nanoribbon.
Definition
ChEBI: A member of the class of furans bearing a hydroxymethyl substituent at the 2-position.
Preparation
Usually prepared from furfural that is obtained by the processing of corncobs; oil obtained by steam distillation of roasted coffee bean meal consists of 50% furfuryl alcohol; prepared industrially by the catalytic reduction of furfural using nickel and Cu-CrO catalysts.
Aroma threshold values
Detection: 1 to 2 ppm.
Taste threshold values
Taste characteristics at 50 ppm: burnt, sweet, caramellic and brown.
Synthesis Reference(s)
Synthesis, p. 246, 1977
Tetrahedron Letters, 33, p. 5417, 1992 DOI: 10.1016/S0040-4039(00)79109-X
General Description
A clear colorless liquid. Flash point 167°F. Boiling point 171°F. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion and skin contact and moderately toxic by inhalation.
Air & Water Reactions
Slightly soluble in water.
Hazard
May react explosively with mineral acids and some organic acids. Toxic by inhalation and skin absorption. Approved for food products. Toxic by skin absorption.
Health Hazard
Inhalation causes headache, nausea, and irritation of nose and throat. Vapor irritates eyes; liquid causes inflammation and corneal opacity. Contact of skin with liquid causes dryness and irritation. Ingestion causes headache, nausea, and irritation of mouth and stomach.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: The product darkens and forms water insoluble material on exposure to air or acids. This reaction is accelerated at elevated temperatures; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Biochem/physiol Actions
Taste at 20-40ppm
Safety Profile
Poison by ingestion, skin contact, and subcutaneous routes.Moderately toxic by inhalation and intraperitoneal routes. Mutation data reported. An eye irritant. Flammable when exposed to heat or flame; can react with oxidtzing materials. Moderate explosion hazard when exposed to heat or flame. Reacts violently with acids (e.g., formic acid, cyanoacetic acid + heat). Ignites on contact with 85% hydrogen peroxide. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Used as a starting monomer in the production of furan resins and used to produce tetrahydro furfural alcohol (THFA).
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
The NTP conducted a 2-year inhalation study on furfuryl alcohol. F344 rats and B6C3F1 mice were exposed to 0, 2, 8, or 32 ppm furfuryl alcohol for 6 h/day, 5 days/week. All rats exposed to 32 ppm died by week 99; survival of all other animals was similar to control animals. There were increased incidences of nasal tumors in the male rats and increased incidences of kidney tubule tumors in male mice. Increased incidences of nonneoplastic lesions of the nose and increased severities of nephropathy were observed in male and female rats and male mice. Nonneoplastic lesions of nose and corneal degeneration occurred in female mice.
Source
Furfuryl occurs naturally in yarrow, licorice, sesame seeds, clove flowers, and tea leaves (Duke, 1992). Also detected in barrel-aged red, white, and model wines. Concentrations ranged from 3.5 mg/L in white wine after 55 wk of aging to 9.6 mg/L after 11 wk of aging (Spillman et al., 1998). Identified as one of 140 volatile constituents in used soybean oils collected from a processing plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
Environmental Fate
Biological. In activated sludge inoculum, following a 20-d adaptation period, 97.3% COD
removal was achieved. The average rate of biodegradation was 41.0 mg COD/g?h (Pitter, 1976).
Chemical/Physical. Easily resinified by acids (Windholz et al., 1983). Furfuryl alcohol will not
hydrolyze because it has no hydrolyzable functional group.
In barrel-aged red, white, and model wines, naturally occurring furfuryl alcohol decreased in
concentration with time. In red wine, furfuryl ethyl ether was identified as a degradation product
after 55 wk of storage. The average percentage decrease of furfuryl alcohol was 73% (Spillman et
al., 1998).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Metal containers involving the transfer of=gallonsor more of ethyl acetate should be grounded and bonded.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of ethyl acetate. Store in containers that are properly labeled with health hazard information and safe handling procedures. Wherever ethyl acetate is used, handled,manufactured, or stored, use explosion-proof electricalequipment and fittings. Furfuryl alcohol must be stored toavoid contact with strong oxidizers (such as chlorine, bromine, and fluorine) and any acid, since violent reactionsoccur. Store in tightly closed containers in a cool, well-ventilated area away from heat. Sources of ignition, such assmoking and open flames, are prohibited where furfurylalcohol is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard. Wherever furfuryl alcohol is used, handled, manufactured, or stored, useexplosion-proof electrical equipment and fittings.
Shipping
UN2874 Furfuryl alcohol, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Distil it under reduced pressure to remove tarry material, shake with aqueous NaHCO3, dry it with Na2SO4 and fractionally distil it under reduced pressure from Na2CO3. It can be further dried by shaking with Linde 5A molecular sieves. [Beilstein 17/3 V 338.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with acids can cause polymerization. Strong reaction with oxidizers. Incompatible with alkaline earth and alkali metals; strong caustics; aliphatic amines; isocyanates, acetaldehyde, benzoyl peroxide; chromic acid, chromium trioxide; cyanoacetic acid; dialkylzincs, dichlorine oxide; ethylene oxide; hydrogen per oxide; isopropyl chlorocarbonate; lithium tetrahydroalumi nate; nitric acid; nitrogen dioxide; pentafluoroguanidine, phosphorus pentasulfide; tangerine oil; triethylaluminum, trii sobutylaluminum. Attacks some plastics, coatings and rubber.
Waste Disposal
Incineration in admixture with a more flammable solvent.
Properties of Furfuryl alcohol
| Melting point: | -29 °C (lit.) |
| Boiling point: | 170 °C (lit.) |
| Density | 1.135 g/mL at 25 °C (lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 0.5 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2491 | FURFURYL ALCOHOL |
| Flash point: | 149 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble |
| form | Liquid |
| pka | 14.02±0.10(Predicted) |
| color | Clear yellow |
| Odor | Mildly irritating. |
| PH | 6 (300g/l, H2O, 20℃) |
| explosive limit | 1.8-16.3%(V) |
| Odor Threshold | 8 ppm |
| Water Solubility | MISCIBLE |
| FreezingPoint | -29℃ |
| Merck | 14,4305 |
| JECFA Number | 451 |
| BRN | 106291 |
| Exposure limits | NIOSH REL: TWA 10 ppm (40 mg/m3), STEL 15 ppm (60 mg/m3), IDLH 75
ppm; OSHA PEL: TWA 50 ppm; ACGIH TLV: TWA 10 ppm, STEL 15 ppm (adopted). |
| CAS DataBase Reference | 98-00-0(CAS DataBase Reference) |
| IARC | 2B (Vol. 119) 2019 |
| NIST Chemistry Reference | 2-Furanmethanol(98-00-0) |
| EPA Substance Registry System | 2-Furanmethanol (98-00-0) |
Safety information for Furfuryl alcohol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Furfuryl alcohol
Furfuryl alcohol manufacturer
Galaxy Laboratories Private Limited
Related products of tetrahydrofuran


You may like
-
 Furfuryl Alcohol 99%View Details
Furfuryl Alcohol 99%View Details -
 Furfuryl alcohol CAS 98-00-0View Details
Furfuryl alcohol CAS 98-00-0View Details
98-00-0 -
 Furfuryl alcohol CAS 98-00-0View Details
Furfuryl alcohol CAS 98-00-0View Details
98-00-0 -
 Furfuryl alcohol CAS 98-00-0View Details
Furfuryl alcohol CAS 98-00-0View Details
98-00-0 -
 Furfuryl Alcohol CASView Details
Furfuryl Alcohol CASView Details -
 Furfuryl Alcohol CASView Details
Furfuryl Alcohol CASView Details -
 Furfuryl alcohol 98% CAS 98-00-0View Details
Furfuryl alcohol 98% CAS 98-00-0View Details
98-00-0 -
 Furfuryl alcohol, puriss CAS 98-00-0View Details
Furfuryl alcohol, puriss CAS 98-00-0View Details
98-00-0