Tetrahydrofurfuryl alcohol
Synonym(s):THFA;Tetrahydrofurfuryl alcohol;Tetrahydro-2-furanmethanol;Tetrahydrofurancarbinol, 2-Hydroxymethyltetrahydrofuran;Oxolan-2-yl methanol
- CAS NO.:97-99-4
- Empirical Formula: C5H10O2
- Molecular Weight: 102.13
- MDL number: MFCD00005372
- EINECS: 202-625-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:58
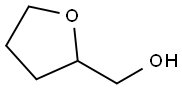
What is Tetrahydrofurfuryl alcohol?
Chemical properties
Tetrahydrofurfuryl alcohol is a colorless to light yellow hygroscopic liquid that has a faint, warm, oily, caramellic odor with a coffee and nut-like flavor at very low levels (0.03 to 1 ppm). Miscible with water, ethanol, ether, acetone, chloroform and benzene, insoluble in paraffin hydrocarbons. It has an extensive history of use as a highly versatile, high purity solvent.
Occurrence
Reported found in shoyu (fermented soya hydrolysate), coffee and fresh mango.
The Uses of Tetrahydrofurfuryl alcohol
Tetrahydrofurfuryl alcohol is used extensively in various industries as a high-purity, water miscible solvent, and as a chemical intermediate. It was used as Solvent for vinyl resins; dyes for leather; chlorinated rubber; cellulose esters; solvent softener for nylon; vegetable oils; coupling agent; organic synthesis.
Preparation
Tetrahydrofurfuryl alcohol is produced by catalytic reduction of furfural with Raney-Ni; also by destructive hydrogenation of lignin.
Definition
ChEBI: Tetrahydrofurfuryl alcohol is a primary alcohol that is methanol in which one of the hydrogens of the methyl group has been replaced by a tetrahydrofuran-2-yl group. It has a role as a protic solvent. It is a primary alcohol and a member of oxolanes.
What are the applications of Application
Tetrahydrofurfuryl alcohol (THFA) is generally regarded as a "green" solvent in industrial applications. THFA is a low cost, biodegradable solvent mainly used as a reactive diluent for epoxy resins and is a good solvent for many of the curative and catalysts used in epoxy formulations. It is also used in the following applications:
Tetrahydrofurfuryl alcohol undergoes chemoselective hydrogenolysis catalyzed by Rh/SiO2 modified with ReOx species to yield 1,5-pentanediol. It undergoes lanthanum-mediated Michael-type addition reaction with maleate to form alkoxybutanedioic acid.
Tetrahydrofurfuryl alcohol was used as refractive-index matching solvent to ensure hard sphere suspension of silica particles for rheological measurements.
Tetrahydrofurfuryl alcohol may be used as an analytical reference standard for the quantification of the analyte in rats and smoke flavoring from rice husk using chromatography techniques.
General Description
Tetrahydrofurfuryl alcohol appears as a clear colorless liquid with a mild odor. Vapors are heavier than air.
Air & Water Reactions
Denser than water and soluble in water.
Reactivity Profile
Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous- carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Flammability and Explosibility
Not classified
Carcinogenicity
Tetrahydro-2-furanmethanol was not mutagenic to S. typhimurium strains TA100, TA1535, TA1537, or TA98 in an in vitro gene mutation assay or to E. coliWP2uvrA/pKM101 with or withoutmetabolic activation. No chromosomal aberrations or polypoidy were observed when tetrahydro-2-furanmethanol was added to cultured Chinese hamster lung cells with or without metabolic activation .
Properties of Tetrahydrofurfuryl alcohol
| Melting point: | -80 °C (lit.) |
| Boiling point: | 178 °C (lit.) |
| Density | 1.054 g/mL at 25 °C (lit.) |
| vapor density | 3.52 (vs air) |
| vapor pressure | 2.3 mm Hg ( 39 °C) |
| refractive index | n |
| FEMA | 3056 | TETRAHYDROFURFURYL ALCOHOL |
| Flash point: | 183 °F |
| storage temp. | Store below +30°C. |
| solubility | acetone: miscible(lit.) |
| form | Liquid |
| pka | 14.44±0.10(Predicted) |
| color | Clear |
| Odor | at 100.00 %. vegetable cauliflower faint warm oily caramel |
| explosive limit | 1.5-9.7%(V) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,9213 |
| JECFA Number | 1443 |
| BRN | 102723 |
| Dielectric constant | 13.48 |
| CAS DataBase Reference | 97-99-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Furanmethanol, tetrahydro-(97-99-4) |
| EPA Substance Registry System | Tetrahydrofurfuryl alcohol (97-99-4) |
Safety information for Tetrahydrofurfuryl alcohol
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tetrahydrofurfuryl alcohol
| InChIKey | BSYVTEYKTMYBMK-UHFFFAOYSA-N |
Tetrahydrofurfuryl alcohol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Tetrahydrofurfuryl Alcohol CAS 97-99-4View Details
Tetrahydrofurfuryl Alcohol CAS 97-99-4View Details
97-99-4 -
 Tetrahydrofurfuryl alcohol, 99% CAS 97-99-4View Details
Tetrahydrofurfuryl alcohol, 99% CAS 97-99-4View Details
97-99-4 -
 Tetrahydrofurfuryl alcohol 98.00% CAS 97-99-4View Details
Tetrahydrofurfuryl alcohol 98.00% CAS 97-99-4View Details
97-99-4 -
 Tetrahydrofurfuryl alcohol 95% CAS 97-99-4View Details
Tetrahydrofurfuryl alcohol 95% CAS 97-99-4View Details
97-99-4 -
 Tetrahydrofurfuryl alcohol CAS 97-99-4View Details
Tetrahydrofurfuryl alcohol CAS 97-99-4View Details
97-99-4 -
 Tetrahydrofurfuryl alcohol CAS 97-99-4View Details
Tetrahydrofurfuryl alcohol CAS 97-99-4View Details
97-99-4 -
 Liquid Tetrahydrofurfurylalcohol, 99%View Details
Liquid Tetrahydrofurfurylalcohol, 99%View Details
97-99-4 -
 Tetrahydrofurfuryl AlcoholView Details
Tetrahydrofurfuryl AlcoholView Details
97-99-4
