Fumes, silica
- CAS NO.:69012-64-2
- Molecular Weight: 0
- EINECS: 273-761-1
- Update Date: 2025-01-06 13:21:43
What is Fumes, silica?
Chemical properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
The Uses of Fumes, silica
None; produced only as a by-product.
Definition
Produced by the volatilization and vaporization of furnace feed materials during the production of ferrosilicon and silicon metals. Consists primarily of silicon dioxide with carbon and trace elements.
What are the applications of Application
Silica-fume forms most of the dust and other particulates in the off-gases produced during the electrothermal production of ferrosilicon (Fe-Si) or silicon (Si). The dust is collected in baghouses and bagged without further treatment. Due to its high surface area, microsilica reacts readily with hydrated calcium silicates forming strong bonds, and for that reason it is sometimes called reactive silica. Therefore the addition of microsilica to hydraulic cements improves their mechanical strength, reduces their permeability, and enhances their workability, cohesiveness, and flowing properties and hence is extensively used as an additive to cements and monolithic refractories.
Health Hazard
Amorphous silica fume exposure is associated with recurrent fever, similar to metal fume fever, and nonprogressive pulmonary changes.More recently, the importance of silica fume particle size on toxicity has been noted.8 Specifically, particles of the ultrafine size range may be expected to have higher toxicity compared with particles of larger size.
Safety Profile
An inhalation hazard. Much less toxic than crystalline forms. Does not cause silicosis. See also other silica entries
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.
Properties of Fumes, silica
| Density | 0.45-0.67[at 20℃] |
| form | Fine white powder with particle
sizes generally below 1mm. This is not
the same as the commercial products “fumes
silica,” “silica gel,” “precipitated silica,” or
“fused silica.” It is formed during the electric
arc production of elemental silicon from quartz, which is reduced to silicon monoxide,
escaping from the furnace and oxidized by air
to silicon dioxide. This condenses to form
spherical particles. For the production of ferrosilicon,
iron metal is added to the charge;
during charging there is also exposure to
crystalline silica. |
| Water Solubility | 135mg/L at 37℃ |
| EPA Substance Registry System | Fumes, silica (69012-64-2) |
Safety information for Fumes, silica
Computed Descriptors for Fumes, silica
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole N-Boc-L-proline methyl ester 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazoleRelated products of tetrahydrofuran
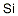



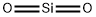
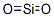
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5