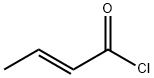
Fumaryl chloride
- CAS NO.:627-63-4
- Empirical Formula: C4H2Cl2O2
- Molecular Weight: 152.96
- MDL number: MFCD00000733
- EINECS: 211-005-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
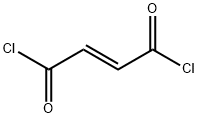
What is Fumaryl chloride?
Chemical properties
Clear yellow liquid
The Uses of Fumaryl chloride
Fumaryl chloride was used in the preparation of high molecular weight poly(propylene fumarate). The photodissociation dynamics of fumaryl chloride was studied. It is used in agrochemicals and pharmaceuticals manufacturing. Also used as an intermediate for dyes, textile auxiliaries and peroxide compounds. It is an acid halide building block.
The Uses of Fumaryl chloride
Chemical intermediate for pharmaceuticals, dyestuffs, and insecticides.
What are the applications of Application
Fumaryl chloride is an acid halide building block
Preparation
Fumaryl chloride has been prepared from fumaric acid and phthaloyl chloride, from maleic acid by the action of thionyl chloride in the presence of zinc chloride, and from maleic anhydride by the use of phthaloyl chloride in the presence of zinc chloride.
Org. Synth. 1940, 20, 51
DOI: 10.15227/orgsyn.020.0051
General Description
Fumaryl chloride appears as a straw colored fuming liquid with a pungent odor. Vapors irritate the eyes and mucous membranes. Corrosive to metals and tissue.
Reactivity Profile
Fumaryl chloride is incompatible with strong oxidizing agents, alcohols, amines and other bases. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Corrosive to eyes and skin.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Moderately toxic by ingestion, inhalation, and skin contact. A skin, eye, and mucous membrane irritant. A corrosive agent. Will react with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits highly toxic fumes of phosgene and HCl.
Properties of Fumaryl chloride
| Melting point: | -2 °C |
| Boiling point: | 161-164 °C(lit.) |
| Density | 1.415 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 165 °F |
| storage temp. | 2-8°C |
| form | Liquid |
| Specific Gravity | 1.415 (20/4℃) |
| color | Clear yellow |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| BRN | 1721342 |
| CAS DataBase Reference | 627-63-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Fumaryl chloride(627-63-4) |
| EPA Substance Registry System | 2-Butenedioyl dichloride, (2E)- (627-63-4) |
Safety information for Fumaryl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fumaryl chloride
| InChIKey | ZLYYJUJDFKGVKB-OWOJBTEDSA-N |
Fumaryl chloride manufacturer
JSK Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 627-63-4 Fumaryl chloride 99%View Details
627-63-4 Fumaryl chloride 99%View Details
627-63-4 -
 Fumaryl Chloride CAS 627-63-4View Details
Fumaryl Chloride CAS 627-63-4View Details
627-63-4 -
 Fumaryl chloride 95% CAS 627-63-4View Details
Fumaryl chloride 95% CAS 627-63-4View Details
627-63-4 -
 Fumaryl chloride CAS 627-63-4View Details
Fumaryl chloride CAS 627-63-4View Details
627-63-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1