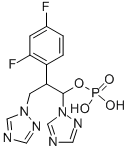
Fosfluconazole
- CAS NO.:194798-83-9
- Empirical Formula: C13H13F2N6O4P
- Molecular Weight: 386.25
- MDL number: MFCD28978011
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
What is Fosfluconazole?
Description
Fosfluconazole is a phosphate prodrug of fluconazole, and it was launched in Japan as an intravenous injection for the treatment of candidiasis and cryptococcosis infections. Fluconazole, a triazole antifungal agent, is a selective inhibitor of fungal cytochrome P450 sterol C-14 alpha-demethylation, and it is widely used for the treatment of patients with serious systemic fungal infections. Fluconazole is marketed in both oral and intravenous formulations, the latter being a dilute (2 mg/ mL) infusion in saline due to the relatively poor water solubility of the drug. In patients needing high doses (>400 mg) of fluconazole, a drawback of the IV formulation is the requirement of a high-volume infusion, which is undesirable in critically ill patients in whom fluid overload must be avoided. Fosfluconazole is a prodrug with approximately 40-fold higher water solubility than fluconazole, thereby achieving a substantial reduction in infusion volume. It is prepared in three steps starting from fluconazole. In the first step, fluconazole is converted to its dibenzyl phosphite derivative by reaction with phosphorous trichloride and benzyl alcohol. Subsequent oxidation of the phosphite to the corresponding phosphate with hydrogen peroxide and cleavage of the benzyl protecting groups by hydrogenolysis affords fosfluconazole. In vitro, fosfluconazole is at least 25-fold less potent than fluconazole against single isolates of Candida species and Cryptococcus neoformans. In vivo, it is rapidly hydrolyzed to fluconazole by phosphatase enzymes and exhibits similar efficacy to fluconazole in experimental models of fungal disease. The hydrolysis potential of fosfluconazole was initially demonstrated in homogenates of kidney, lung and liver of rat, dog, and human. Subsequently, in clinical trials with healthy volunteers (n=24), fosfluconazole was shown to hydrolyze rapidly and almost completely to provide a 97% mean bioavailability of fluconazole. Less than 1% of the administered dose of fosfluconazole was excreted unchanged in the urine, with the majority (85.6%) of the dose eliminated as fluconazole. The terminal half-life was about 2.3 hours, and the volume of distribution was 0.2 L/kg. The time to reach steady state drug levels with 500 mg daily dose was about 10 days, which could be shortened to 3 days by administering loading doses of 1000 mgs on days 1 and 2 followed by 500 mg daily. Further studies showed that hepatic or renal impairment did not significantly alter the pharmacokinetic profile of fosfluconazole. In phase III studies in patients with deep-seated mycosis due to Candida or Cryptococcus (n=160), a 2-day loading dose regimen of fosfluconazole provided efficacy range of 73.8% (in Japanese patients) to 91.7% (patients of non-Japanese origin). The adverse events seen in these trials were similar to those previously known with fluconazole therapy and included rash (3.1%), abnormal liver function values (2.5%), asthma (1.9%), and lightheadedness (1.9%).
Originator
Pfizer (Japan)
Definition
ChEBI: Fosfluconazole is a member of triazoles, a triazole antifungal drug and a conazole antifungal drug. It has a role as a prodrug. It is functionally related to a fluconazole.
brand name
Prodif
Properties of Fosfluconazole
| Melting point: | 223-224° |
| Boiling point: | 701.5±70.0 °C(Predicted) |
| Density | 1.70±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 6.2 mg/mL (16.05 mM) |
| form | Solid |
| pka | 1.44±0.10(Predicted) |
| color | White to off-white |
Safety information for Fosfluconazole
Computed Descriptors for Fosfluconazole
Fosfluconazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 194798-83-9 Fosfluconazol 98%View Details
194798-83-9 Fosfluconazol 98%View Details
194798-83-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8