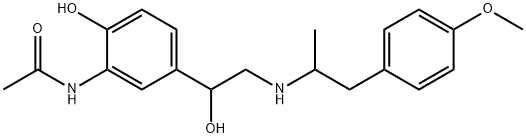
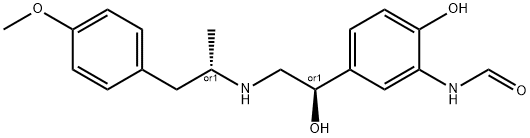
(R,R)-Formoterol
- CAS NO.:67346-49-0
- Empirical Formula: C19H24N2O4
- Molecular Weight: 344.4
- MDL number: MFCD00864141
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:17
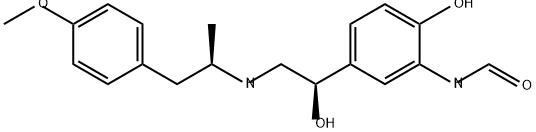
What is (R,R)-Formoterol?
Absorption
In patients with COPD, the mean peak plasma concentration (Cmax) and AUC0-12h following twice daily administration for 14 days were 4.3 pg/mL and 34.5 pg.hr/mL, respectively. The time to peak plasma concentration (Tmax) was approximately 0.5 hours.
Toxicity
A death was reported in dogs after a single oral dose of 5 mg/kg (approximately 4500 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). As with all inhaled sympathomimetic medications, cardiac arrest and even death may be associated with an overdose. Arformoterol should not be used more often or at higher doses than recommended, or conjunction with other medications containing long-acting beta2-agonists.
Description
Arformoterol, a selective long-acting b2-agonist, was launched as an inhalation solution for treatment of bronchoconstriction associated with COPD. It is the active (R,R)-enantiomer of the previously marketed b2-agonist formoterol, which is registered as a racemic mixture. Similar to other b2-agonists, the mechanism of action of formoterol and arformoterol involves activation of adenyl cyclase, leading to increased production of cyclic adenosine monophosphate (cAMP) via ATP. Increased levels of intracellular cAMP result in relaxation of the bronchial smooth muscle, and also prevent mast cells from releasing inflammatory mediators. Arformoterol has high binding affinity for the human b2 receptor (Kd 2.9 nM) and approximately 39-fold selectivity over the b1 receptor (Kd 113 nM). It is W1,000 times more potent b2 ligand than the corresponding (S,S)-enantiomer (Kd 3100 nM), and twice as potent as racemic formoterol (Kd 5.2 nM). Additionally, arformoterol acts as a full or nearly full agonist of the b2 receptor, whereas the (S,S)-enantiomer acts as an inverse agonist. Therefore, the (R,R)-enantiomer may account exclusively for the activation of b2 receptors by the racemic mixture.
Originator
Sepracor (US)
The Uses of (R,R)-Formoterol
(R,R)-Formoterol (Arformoterol) (CAS# 67346-49-0) is aused in the methods for treating chronic obstructive pulmonary disease by administration of a bronchodilator using a nebulizer.
The Uses of (R,R)-Formoterol
Anti-asthmatic and bronchodilator.
Background
Arformoterol is a bronchodilator. It works by relaxing muscles in the airways to improve breathing. Arformoterol inhalation is used to prevent bronchoconstriction in people with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. The use of arformoterol is pending revision due to safety concerns in regards to an increased risk of severe exacerbation of asthma symptoms, leading to hospitalization as well as death in some patients using long-acting beta agonists for the treatment of asthma.
Indications
Arformoterol is indicated in the maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.
Definition
ChEBI: An N-[2-hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]formamide in which both of the stereocentres have R configuration. The active enantiomer of formoterol, it is administered by inhalation generally as the tartrate salt) as a direct-acting sympathomimetic and bronchodilator for the treatment of chronic obstructive pulmonary disease (any progressive respiratory disease that makes it harder to breathe over time, such as chronic bronchitis and emphysema).
brand name
Brovana
Pharmacokinetics
Arformoterol, the active (R,R)-enantiomer of formoterol, is a selective long-acting β2-adrenergic receptor agonist (beta2-agonist) that has two-fold greater potency than racemic formoterol (which contains both the (S,S) and (R,R)-enantiomers). The (S,S)-enantiomer is about 1,000-fold less potent as a β2-agonist than the (R,R)-enantiomer. Arformoterol seems to have little or no effect on β1-adrenergic receptors.
Metabolism
Arformoterol was almost entirely metabolized following oral administration of 35 mcg of radiolabeled arformoterol in eight healthy subjects. Direct conjugation of arformoterol with glucuronic acid was the major metabolic pathway. O-Desmethylation is a secondary route catalyzed by the CYP enzymes CYP2D6 and CYP2C19.
Properties of (R,R)-Formoterol
| Melting point: | 73-75 °C |
| Boiling point: | 603.2±55.0 °C(Predicted) |
| Density | 1.233±0.06 g/cm3(Predicted) |
| solubility | 25℃: DMSO |
| form | Powder |
| pka | 8.95±0.50(Predicted) |
| color | White to off-white |
Safety information for (R,R)-Formoterol
Computed Descriptors for (R,R)-Formoterol
(R,R)-Formoterol manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Formamide, N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, [R-(R*,S*)]-](https://img.chemicalbook.in/CAS/GIF/67346-51-4.gif)
You may like
-
 67346-49-0 Arformoterol 98%View Details
67346-49-0 Arformoterol 98%View Details
67346-49-0 -
 67346-49-0 98%View Details
67346-49-0 98%View Details
67346-49-0 -
 Arformoterol 99%View Details
Arformoterol 99%View Details
67346-49-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1