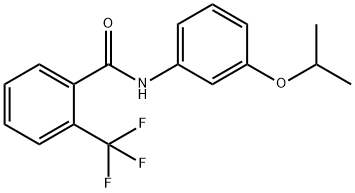
Flutolanil
Synonym(s):N-(3-Isopropoxyphenyl)-2-(trifluoromethyl)benzamide
- CAS NO.:66332-96-5
- Empirical Formula: C17H16F3NO2
- Molecular Weight: 323.31
- MDL number: MFCD00176914
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
What is Flutolanil?
The Uses of Flutolanil
Flutolanil is used to control Basidiomycetes diseases in rice, cereals, sugar beet and other crops.
The Uses of Flutolanil
Agricultural fungicide.
The Uses of Flutolanil
Flutolanil is a fungicide that has been used for controlling brown patch on creeping bentgrass fairways.
Definition
ChEBI: Flutolanil is a member of the class of benzamides, obtained by formal condensation of the carboxy group of 2-(trifluoromethyl)benzoic acid with the amino group of 3-(ispropyloxy)aniline. A fungicide used to control a range of pathogens especially Rhizoctonia spp. on rice, turf and other crops. It has a role as an EC 1.3.5.1 [succinate dehydrogenase (quinone)] inhibitor and an antifungal agrochemical. It is a member of benzamides, an aromatic ether, a member of (trifluoromethyl)benzenes and a benzanilide fungicide.
What are the applications of Application
Flutolanil 40SC Fungicide is a systemic fungicide for control of Basidiomycete diseases on turf. This product has shown excellent safety on Kentucky bluegrass, annual bluegrass, annual and perennial ryegrass, red fescue, tall fescue, bentgrass, Bermudagrass, zoysiagrass, and St. Augustine grass. Flutolanil 40SC Fungicide may be tank mixed with other labeled fungicides.
Trade name
MONCUT
Safety Profile
Low toxicity by ingestion, skincontact, intraperitoneal, and subcutaneous routes. Whenheated to decomposition it emits toxic vapors of NOx andF??.
Metabolic pathway
Flutolanil is an analogue of mepronil in which the methyl group is replaced by trifluoromethyl. Both compounds have systemic activity. This change in structure should render flutolanil more biostable by hindering hydrolysis and removing the option of methyl hydroxylation and further oxidation. This seems to be borne out in practice in that most of the metabolism of flutolanil occurs via O-dealkylation and aryl hydroxylation. Hydrolysis has not been detected.
Degradation
Flutolanil is a stable arylamide with no particularly weak link in its comparatively
simple chemistry. It is stable over the pH range 3-11 and it is
stable to heat (PM). It is stable in sunlight (PM) but it was slowly
degraded in 50% aqueous ethanol solution irradiated with a high pressure
mercury lamp whilst bubbling oxygen through the solution (Tsao and Eto,
1991). The study was conducted using non-radiolabelled compound. No
degradation occurred in the absence of oxygen. Even under these conditions,
the addition of photosensitisers was required to give a reasonable amount of breakdown. With 5% acetone in the solution, 20% degradation
was obtained in 8 hours. Almost no decomposition occurred on a glass
surface in 8 hours unless a sensitiser (e.g. benzophenone) was added. This
gave 40% decomposition.
The products in solution and on surfaces were different, as shown in
Scheme 1. The major product (80%) in solution was 2-(trifluoromethyl)-
benzamide (2). The benzoic acid (3) was identified as a minor product.
The N-ethoxycarbonyl derivative (4) was due to reaction with the solvent.
Amide bond cleavage was postulated to occur via oxidation in the aniline
ring (Tsao and Eto, 1991; Yumita et al., 1984). The resulting phenolic
products and anilines were converted into unidentified polar polymers.
No product 2 was obtained by irradiation on a glass surface (Tsao and
Eto, 1991). Under these conditions 3'-hydroxy-2-(trifluoromethyl)benzanilide
(5), i.e. dealkylated flutolanil, and a rearrangement product (6)
were the main products. Flutolanil is therefore an extremely stable
compound which undergoes slow photo-oxidation rather than aqueous
photolysis.
Mode of action
Flutolanil is a SDHI (Succinate-dehydrogenase inhibitor) with narrow spectrum. Flutolanil has inhibitory effect against development of each step of the infection cycle. Inhibition of invasion from sclerotium and mycelial growth result in preventive and curative effect in infection, respectively。
Properties of Flutolanil
| Melting point: | 108° (Araki, Yabutani); mp 104-105° (Araki, 1985) |
| Boiling point: | 339.1±42.0 °C(Predicted) |
| Density | 1.2463 (estimate) |
| vapor pressure | 6.5 x 10-6 Pa (25 °C) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.44±0.70(Predicted) |
| Water Solubility | 6.53 mg l-1 (20 °C) |
| color | White to Pale Orange |
| EPA Substance Registry System | Flutolanil (66332-96-5) |
Safety information for Flutolanil
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Flutolanil
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Flutolanil CAS 66332-96-5View Details
Flutolanil CAS 66332-96-5View Details
66332-96-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1