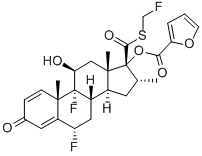
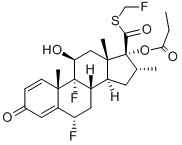
Fluticasone
- CAS NO.:90566-53-3
- Empirical Formula: C22H27F3O4S
- Molecular Weight: 444.51
- MDL number: MFCD01862254
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
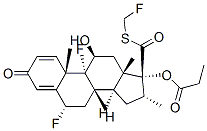
What is Fluticasone?
Absorption
Intranasal exposure of Fluticasone furoate results in patients swallowing a larger portion of the dose. However, absorption is poor and metabolism is high, therefore there is negligible systemic exposure with a nasal bioavailability of 0.50% and oral bioavialability of 1.26%. Inhaled bioavailability is 13.9%. A study of 24 healthy Caucasian males showed an inhaled bioavailability of 6.3-18.4%.
Intranasal bioavailability of Fluticasone propionate is <2%, and oral bioavailability is <1%. Intranasal exposure results in the majority of the dose being swallowed. Topical absorption of Fluticasone propionate is very low but can change depending on a number of factors including integrity of the skin and the presence of inflammation or disease. A study of 24 healthy Caucasian males showed an inhaled bioavailability of 9.0%.
Toxicity
Fluticasone furoate administered nasally may be associated with adrenal suppression or an increase in QTc interval though the association has not been well demonstrated in studies. Fluticasone furoate requires no dosage adjustment in renal impairment but must be used in caution in hepatic impairment due to the elimination mechanisms. Fluticasone furoate is not associated with carcinogenicity, mutagenicity, or impairment of fertility. There are no well controlled studies in pregnancy or lactation though animal studies have shown teratogenicity and hypoadrenalism in the offspring of treated mothers and other corticosteroids are known to be excreted in breast milk. Generally, there are no reported adverse effects with fluticasone in pregnancy. Pediatric patients should be given the lowest possible dose and monitored for reduction in growth velocity. There is insufficient evidence to determine whether geriatric patients respond differently to other patients. Systemic exposure may be 27-49% higher in Japanese, Korean, and Chinese patients compared to Caucasian patients. Caution should be exercised in these patients and the benefit and risk should be assessed before deciding on a treatment.
Fluticasone propionate's use in specific populations has not been well studied. Fluticasone propionate is not carcinogenic, mutagenic, or clastogenic, nor did it affect fertility in animal studies. Subcutaneous Fluticasone propionate has been shown to produce teratogenic effects in rats though oral administration does not. Generally, there are no reported adverse effects with fluticasone in pregnancy. Fluticasone propionate in human milk may cause growth suppression, effects on endogenous corticosteroid production, or other effects. Pediatric patients treated with Fluticasone propionate ointment experienced adrenal suppression. Geriatric patients treated with Fluticasone propionate did not show any difference in safety or efficacy compared to other patient groups, though older patients may be more sensitive to adverse effects. There is no difference in the clearance of Fluticasone propionate across genders or race. Patients with hepatic impairment should be closely monitored due to the elimination mechanism.
The Uses of Fluticasone
Fluticasone is an inhaled corticosteroid used in the treatment of respiratory diseases.
The Uses of Fluticasone
therapeutic for asthma and allergic rhinitis Flonase
Indications
Fluticasone's 2 esters are indicated as inhalers for the treatment and management of asthma by prophylaxisas well as inflammatory and pruritic dermatoses. A Fluticasone propionate nasal spray is indicated for managing nonallergic rhinitis while the Fluticasone furoate nasal spray is indicated for treating season and perennial allergic rhinitis.
Background
Fluticasone is a synthetic glucocorticoid available as 2 esters, Fluticasone furoate and Fluticasone propionate. These drugs are available as inhalers, nasal, sprays, and topical treatments for various inflammatory indications. Fluticasone propionate was first approved in 1990 and Fluticasone furoate was approved in 2007.
Definition
ChEBI: A trifluorinated corticosteroid used in the form of its propionate ester for treatment of allergic rhinitis.
brand name
Cutivate (Altana); Flonase (GlaxoSmithKline); Flovent (GlaxoSmithKline).
Pharmacokinetics
Systemically, in vitro experiments show Fluticasone furoate activates glucocorticoid receptors, inhibits nuclear factor kappa b, and inhibits lung eosinophilia in rats. Fluticasone propionate performs similar activity but is not stated to affect nuclear factor kappa b. Fluticasone propionate as a topical formulation is also associated with vasoconstriction in the skin.
Metabolism
Fluticasone furoate and Fluticasone propionate are cleared from hepatic metabolism by cytochrome P450 3A4. Both are hydrolysed at the FIVE-S-fluoromethyl carbothioate group, forming an inactive metabolite.
Properties of Fluticasone
| Melting point: | 237-239 °C |
| Boiling point: | 553.2±50.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly) |
| form | Solid |
| pka | 11.66±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic, Unstable in Solution |
| CAS DataBase Reference | 90566-53-3(CAS DataBase Reference) |
Safety information for Fluticasone
Computed Descriptors for Fluticasone
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![(7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol](https://img.chemicalbook.in/CAS2/GIF/153004-31-0.gif)


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4