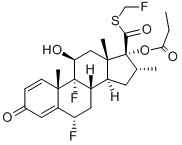
Fluticasone propionate
Synonym(s):(6α,11β,16α,17α)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid S-(fluoromethyl) ester;Fluticasone propionate
- CAS NO.:80474-14-2
- Empirical Formula: C25H31F3O5S
- Molecular Weight: 500.57
- MDL number: MFCD00866007
- EINECS: 617-082-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
What is Fluticasone propionate?
Absorption
Intranasal bioavailability of fluticasone propionate is <2%, and oral bioavailability is <1%. Intranasal exposure results in the majority of the dose being swallowed. Topical absorption of fluticasone propionate is very low but can change depending on a number of factors including integrity of the skin and the presence of inflammation or disease. A study of 24 healthy Caucasian males showed an inhaled bioavailability of 9.0%.
Toxicity
Fluticasone propionate's use in specific populations has not been well studied. Fluticasone propionate is not carcinogenic, mutagenic, or clastogenic, nor did it affect fertility in animal studies. Subcutaneous fluticasone propionate has been shown to produce teratogenic effects in rats though oral administration does not. Generally, there are no reported adverse effects with fluticasone in pregnancy. Fluticasone propionate in human milk may cause growth suppression, effects on endogenous corticosteroid production, or other effects. Pediatric patients treated with fluticasone propionate ointment experienced adrenal suppression. Geriatric patients treated with fluticasone propionate did not show any difference in safety or efficacy compared to other patient groups, though older patients may be more sensitive to adverse effects. There is no difference in the clearance of fluticasone propionate across genders or race. Patients with hepatic impairment should be closely monitored due to the elimination mechanism.
Description
Fluticasone propionate is claimed to exert a topical effect on the lungs without significant systemic effects, due to its low systemic bioavailability.
Chemical properties
Fluticasone propionate is a white to off-white powder with a molecular weight of 500.6, and 17 the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in 18 dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Originator
Glaxo (United Kingdom)
The Uses of Fluticasone propionate
Fluticasone Propionate is a derivative of Flumethasone, a glucocorticoid that has antiallergic, anti-asthmatic, and anti-inflammatory properties. It is used in the treatment of seasonal rhinitis and asthma. Compared to beclomethasone dipropionate, fluticasone propionate is reported to have a comparable side effect profile, but twice the activity in the treatment of asthma.
The Uses of Fluticasone propionate
Fluticasone propionate, a medium-potency synthetic corticosteroid, is used topically to relieve inflammatory and pruritic symptoms of dermatoses and psoriasis, intranasally to manage symptoms of allergic and non-allergic rhinitis, and orally for the treatment of asthma. Fluticasone Proprionate is marketed under several different brand names such as Flonase(R). Fluticasone propionate is also available as a combination product of Azelastine Hydrochloride and Fluticasone Propionate called Dymista(TM). Dymista(TM) is indicated in patients over 12 years old for symptomatic relief of seasonal allergic rhinitis.
Background
Fluticasone propionate is a synthetic glucocorticoid. These drugs are available as inhalers, nasal, sprays, and topical treatments for various inflammatory indications. Fluticasone propionate was first approved in 1990.
Indications
Fluticasone propionate is indicated as an inhaler for the treatment and management of asthma by prophylaxisas well as inflammatory and pruritic dermatoses. Fluticasone propionate nasal spray is indicated for managing allergic and nonallergic rhinitis.
What are the applications of Application
Fluticasone propionate is a high-affinity, selective glucocorticoid receptor agonist. Fluticasone propionate belongs to the group of corticosteroid drugs. It is available in dosage forms such as creams or ointments, nasal sprays and inhalers. It has anti-inflammatory properties and helps to block substances associated with the body's reaction to allergens, thus reducing nasal inflammation overall, which in turn reduces the symptoms of a runny nose, nasal congestion and itchy, watery eyes.
Definition
ChEBI: Fluticasone propionate is a trifluorinated corticosteroid that consists of 6alpha,9-difluoro-11beta,17alpha-dihydroxy-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-16-methyl-3-oxoandrosta-1,4-diene bearing a propionyl substituent at position 17; has anti-inflammatory, anti-asthmatic and anti-allergic activity. It has a role as an anti-allergic agent, an anti-asthmatic drug, an anti-inflammatory drug, a dermatologic drug, a bronchodilator agent and an adrenergic agent. It is a corticosteroid, a steroid ester, an 11beta-hydroxy steroid, a propanoate ester, a fluorinated steroid, a thioester and a 3-oxo-Delta(1),Delta(4)-steroid. It derives from a fluticasone. It derives from a hydride of an androstane.
Preparation
Fluticasone propionate, synthesized by Glaxo Wellcome and launched in 1993, is a trifluorinated glucocorticosteroid. It shows good topical antiinflammatory activity and is commonly used as a safe and effective inhaled treatment for asthma and allergic rhinitis.
Fluticasone propionate can be synthesized from 9 by using BrCH2F,2a ClCH2F,5a or S-(monofluoromethyl) diarylsulfonium tetrafluoroborate 5f,g directly. Using BrCH2F can get an ideal yield; however, BrCH2F is costly and will destroy to the ozone layer. In addition, 9 reacted with BrCH2Cl or Br2CH2 and then by an anion exchange with AgF,2c,5e KF, or tetrabutylammonium fluoride5b to afford 1 in a low yield.
Improved Synthesis of Fluticasone Propionate
Manufacturing Process
A solution of S-iodomethyl-6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo17α-propionyloxyandrosta-1,4-diene-17β-carbothioate (310 mg) in acetonitrile (10 ml) was stirred with silver fluoride (947 mg) for 3 days at room temperature in the dark. Ethyl acetate (100 ml) was added and the mixture was filtered through kieselguhr. The filtrate was washed successively with 2 N hydrochloric acid, water, saturated brine, then dried. The solvent was removed and the residue was subjected to column chromatography in chloroform then chloroform-acetone (19:1). The product was eluted with ethyl acetate and crystallised on concentration of the solution to give S-fluoromethyl 6α,9αdifluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene17β-carbothioate (0.075 g); melting point 272-273°C (dec.), [α]D= +30° (c 0.35).
brand name
Cutivate (Altana); Flonase (GlaxoSmithKline); Flovent (GlaxoSmithKline);Flixonase.
Therapeutic Function
Glucocorticoid
General Description
Fluticasone Propionate is the propionate salt form of fluticasone, a synthetic trifluorinated glucocorticoid receptor agonist with antiallergic, antiinflammatory and antipruritic effects. Binding and activation of the glucocorticoid receptor results in the activation of lipocortin that in turn inhibits cytosolic phospholipase A2, which triggers cascade of reactions involved in synthesis of inflammatory mediators, such as prostaglandins and leukotrienes. Secondly, mitogen-activated protein kinase (MAPK) phosphatase 1 is induced, thereby leads to dephosphorylation and inactivation of Jun N-terminal kinase directly inhibiting c-Jun mediated transcription. Finally, transcriptional activity of nuclear factor (NF)-kappa-B is blocked, thereby inhibits the transcription of cyclooxygenase 2, which is essential for prostaglandin production.
Biological Activity
Fluticasone propionate is a high affinity, selective glucocorticoid receptor agonist (Kd = 0.5 nM). Potently stimulates glucocorticoid receptor-mediated transactivation of gene expression and enhances human eosinophil apoptosis (EC50 = 3.7 nM) in vitro. Inhibits mast cell accumulation in nasal mucosa following topical administration. Lipophilic anti-inflammatory agent with low oral bioavailability. Also potentiates KV1 channels (EC50 = 37 nM).
Mechanism of action
Fluticasone propionate has a unique C-20 thioflouromethyl group, and that, in combination with the 17α-propionate ester, gives it 36-fold the glucocorticoid receptor affinity as compared to beclomethasone dipropionate and twofold the affinity as compared to budesonide. The 9α-flouro group increases both glucocorticoid and mineralocorticoid activities, and the 6α-flouro group enhances only the glucocorticoid action. Studies have determined that the effectiveness of inhaled fluticasone propionate results from a local rather than a systemic effect.
Pharmacokinetics
Systemically, fluticasone propionate activates glucocorticoid receptors, and inhibits lung eosinophilia in rats. Fluticasone propionate as a topical formulation is also associated with vasoconstriction in the skin.
Side Effects
In controlled US studies, more than 3,300 patients with seasonal allergic, perennial allergic, or perennial nonallergic rhinitis received treatment with intranasal fluticasone propionate. In general, adverse reactions in clinical studies have been primarily associated with irritation of the nasal mucous membranes, and the adverse reactions were reported with approximately the same frequency by patients treated with the vehicle itself. The complaints did not usually interfere with treatment. Less than 2% of patients in clinical trials discontinued because of adverse events; this rate was similar for vehicle placebo and active comparators.
Systemic corticosteroid side effects were not reported during controlled clinical studies up to 6 months’ duration with FLONASE Nasal Spray. If recommended doses are exceeded, however, or if individuals are particularly sensitive or taking FLONASE Nasal Spray in conjunction with administration of other corticosteroids, symptoms of hypercorticism, e.g., Cushing syndrome, could occur.
PRESCRIBING INFORMATION
Veterinary Drugs and Treatments
While there are topical forms of fluticasone, most veterinary interests are in the inhaled versions of the drug. The aerosol for pulmonary inhalation appears to be effective in treating feline asthma, recurrent airway obstruction (RAO, heaves) or inflammatory airway disease (IAD) in horses, and dogs with chronic tracheobronchial disease. While the majority of small animal use has been with fluticasone, there are several other aerosol corticosteroids for inhalation (beclomethasone dipropionate, flunisolide, and triamcinolone acetonide) that theoretically could be used for the same purpose. The nasal inhalation corticosteroid products may be useful for allergyrelated chronic rhinosinusitis in cats and dogs.
Metabolism
Fluticasone propionate is cleared from hepatic metabolism by cytochrome P450 3A4. Fluticasone propionate is hydrolysed at the FIVE-S-fluoromethyl carbothioate group, forming an inactive metabolite.
Metabolism
Interestingly, less than 1% of the swallowed dose is bioavailable, in contrast to the fact that the majority of the inhaled dose is systemically available and highly protein bound. Fluticasone propionate is not a prodrug and is extensively metabolized by the liver. The only detectable metabolite is the 17β-carboxylic acid derived from CYP3A4 oxidation. This metabolite has 2,000-fold less affinity for the glucocorticoid receptor than the parent drug. Elimination is through both the feces and the urine, with the relative amounts determined by the route of administration. Fluticasone propionate is available in aerosol and powder inhalation formulations.
Side Effects
The most common side effects of Fluticasone propionate include: headache, nausea and vomiting, sore throat, nosebleed, difficulty breathing, cough, burning or itching in the nose. Severe allergic reactions are rare and manifest as symptoms such as sudden swelling of the face or tongue, rash, difficulty breathing, or feeling faint.
Storage
Room temperature
Dosage
Adults: The recommended starting dosage in adults is 2 sprays (50 mcg of fluticasone propionate each) in each nostril once daily (total daily dose, 200 mcg). The same dosage divided into 100 mcg given twice daily (e.g., 8 a.m. and 8 p.m.) is also effective. After the first few days, patients may be able to reduce their dosage to 100 mcg (1 spray in each nostril) once daily for maintenance therapy. Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of 200 mcg once daily effective for symptom control (see Clinical Trials). Greater symptom control may be achieved with scheduled regular use.
Properties of Fluticasone propionate
| Melting point: | 275 °C |
| Boiling point: | 568.3±50.0 °C(Predicted) |
| alpha | D +30° (c = 0.35) |
| Density | 1.32±0.1 g/cm3(Predicted) |
| refractive index | 31 ° (C=1, Dioxane) |
| storage temp. | Store at RT |
| solubility | DMSO: ≥10 mg/mL |
| form | solid |
| pka | 12.53±0.70(Predicted) |
| color | white |
| Merck | 14,4211 |
| CAS DataBase Reference | 80474-14-2(CAS DataBase Reference) |
| EPA Substance Registry System | Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-,S-(fluoromethyl) ester, (6.alpha.,11.beta.,16.alpha.,17.alpha.)- (80474-14-2) |
Safety information for Fluticasone propionate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P281:Use personal protective equipment as required. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Fluticasone propionate
| InChIKey | WMWTYOKRWGGJOA-CENSZEJFSA-N |
Fluticasone propionate manufacturer
Pharma Links
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Fluticasone Propionate 98%View Details
Fluticasone Propionate 98%View Details -
 Fluticasone Propionate 99%View Details
Fluticasone Propionate 99%View Details -
 Fluticasone propionate 99%View Details
Fluticasone propionate 99%View Details -
 Fluticasone Propionate 99%View Details
Fluticasone Propionate 99%View Details -
 FLUTICASONE PROPIONATE 95-99%View Details
FLUTICASONE PROPIONATE 95-99%View Details
80474-14-2 -
 Fluticasone Propionate CAS 80474-14-2View Details
Fluticasone Propionate CAS 80474-14-2View Details
80474-14-2 -
 Fluticasone Propionate (IP)View Details
Fluticasone Propionate (IP)View Details
80474-14-2 -
 99% Fluticasone Propionate USP/EP, Jai Radhe Sales, 1KgView Details
99% Fluticasone Propionate USP/EP, Jai Radhe Sales, 1KgView Details
80474-14-2
