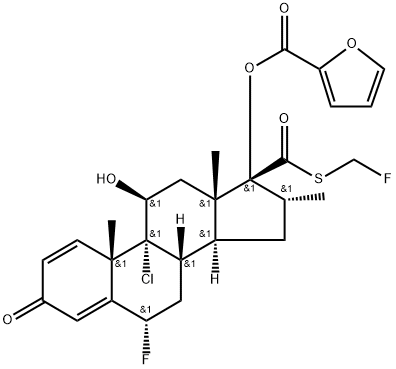
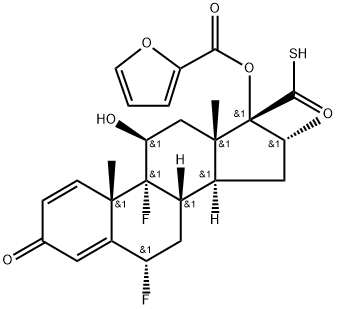
FLUTICASONE FUROATE
Synonym(s):6α,9α-Difluoro-17α-[(2-furanylcarbonyl)oxy]-11β-hydroxy-6α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioic acid S-fluoromethyl ester;6a,9a-Difluoro-17a-[(2-furanylcarbonyl)oxy]-11β-hydroxy-16a-methyl-3-oxoandrosta-1,4-diene-17β-carbothioic acid S-fluoromethyl ester;Veramyst
- CAS NO.:397864-44-7
- Empirical Formula: C27H29F3O6S
- Molecular Weight: 538.58
- MDL number: MFCD09954122
- EINECS: 222-789-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
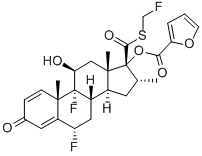
What is FLUTICASONE FUROATE?
Absorption
Fluticasone furoate plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 0.5 to 1 hour. Absolute bioavailability of fluticasone furoate when administrated by inhalation was 13.9%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose is low (approximately 1.3%) due to extensive first-pass metabolism. Systemic exposure (AUC) in subjects with asthma was 26% lower than observed in healthy subjects. Following repeat dosing of inhaled fluticasone furoate, steady state was achieved within 6 days with up to 2.6-fold accumulation. Intranasal exposure of fluticasone furoate also results in patients swallowing a larger portion of the dose.
Toxicity
Fluticasone furoate administered nasally may be associated with adrenal suppression or an increase in QTc interval though the association has not been well demonstrated in studies. Fluticasone furoate requires no dosage adjustment in renal impairment but must be used with caution in hepatic impairment due to the elimination mechanisms. Fluticasone furoate is not associated with carcinogenicity, mutagenicity, or impairment of fertility. There are no well-controlled studies in pregnancy or lactation though animal studies have shown teratogenicity and hypoadrenalism in the offspring of treated mothers and other corticosteroids are known to be excreted in breast milk. Generally, there are no reported adverse effects with fluticasone in pregnancy. Pediatric patients should be given the lowest possible dose and monitored for a reduction in growth velocity. There is insufficient evidence to determine whether geriatric patients respond differently to other patients. Systemic exposure may be 27-49% higher in Japanese, Korean, and Chinese patients compared to Caucasian patients. Caution should be exercised in these patients and the benefit and risk should be assessed before deciding on a treatment.
Description
Fluticasone furoate is a new corticosteroid derivative launched as a
nasal spray for the treatment of seasonal and perennial allergic rhinitis in adults
and in children aged ≥2 years. It is structurally closely related to the previously
marketed intranasal corticosteroid fluticasone propionate (FP). Both of these
steroids contain the unusual S-fluoromethyl carbothioate group, which confers
high lipophilicity and hence enhanced binding and retention of the drug by the
nasal tissue. Additionally, the carbothioate group rapidly undergoes first-pass
metabolism by CYP3A4, thus minimizing systemic exposure of the parent drug.
Fluticasone furoate is a potent ligand for the glucocorticoid receptor (GR), with
a relative receptor affinity (RRA) of 2,989 with reference to dexamethasone RRA
of 100.
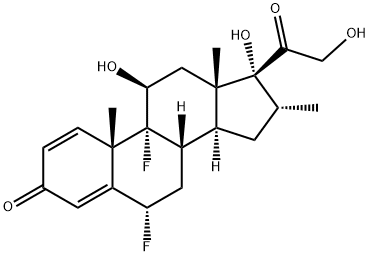
Following intranasal administration, most of the dose is eventually swallowed and undergoes incomplete absorption and extensive firstpass metabolism in the liver and gut, resulting in negligible systemic exposure. Pharmacokinetic studies using oral solution dosing and intravenous dosing show that at least 30% of fluticasone furoate is absorbed and then rapidly cleared from plasma. The most common adverse reactions (W1% incidence) included headache, epistatix, sinus and throat pain, nasal ulceration, back pain, pyrexia, and cough. Fluticasone furoate is chemically derived starting from a readily available corticosteroid, 6a,9a-difluoro-11b,17a-dihydroxy-16a-methyl-3-oxoandrosta-1,4-diene-17b-carboxylic acid, by first converting the carboxylic acid group to the corresponding carbothioic acid via activation with carbonyl diimidazole followed by reaction with hydrogen sulfide gas. Subsequently, selective acylation of the 17a-hydroxyl group with 2-furoyl chloride and alkylation of the 17b-carbothioic acid group with bromofluoromethane under basic conditions provides fluticasone furoate.
Description
Fluticasone furoate is a synthetic glucocorticoid. It is selective for the glucocorticoid receptor over the mineralocorticoid, progesterone, and androgen receptors in reporter assays (EC50s = 0.03, 23.4, 0.9, and >10,000 nM, respectively), as well as estrogen receptor α (ERα) and ERβ in scintillation proximity assays (EC50s = >10,000 nM for both). Fluticasone furoate reduces LPS-induced increases in TNF-α production in human peripheral blood mononuclear cells (PBMCs) with an EC50 value of 0.12 nM. It decreases S. aureus enterotoxin-induced increases in IFN-γ, IL-2, IL-5, IL-17, and TNF-α levels in patient-derived nasal polyp tissue when used at a concentration of 100 nM. Intrathecal administration of fluticasone furoate (30 μg/animal) reduces ovalbumin-induced increases in bronchoalveolar lavage fluid (BALF) eosinophil infiltration in a rat model of allergic inflammation. Formulations containing fluticasone furoate have been used in the treatment of seasonal allergies.
Chemical properties
White to Off-White Solid
Originator
GSK (US)
The Uses of FLUTICASONE FUROATE
Fluticasone-d3 Furoate is an isotope labelled form of Fluticasone Furoate (F599510). Fluticasone Furoate is a synthetic corticosteroid derived from fluticasone for treatment of seasonal allergic rhinitis.
The Uses of FLUTICASONE FUROATE
It is one of the newest
Background
Fluticasone furoate is a synthetic glucocorticoid available as an inhaler and nasal spray for various inflammatory indications. Fluticasone furoate was first approved in 2007.
Indications
Fluticasone furoate is indicated for once-daily maintenance (i.e. prophylactic) treatment of asthma in patients ≥5 years old. Fluticasone furoate is available in two combination medications - one in combination with vilanterol and one in combination with both vilanterol and umeclidinium- which are both indicated for the management of chronic obstructive pulmonary disease (COPD) and for the treatment of asthma in patients ≥18 years old for the vilanterol-umeclidinium-fluticasone product and ≥5 years old for the vilanterol-fluticasone product.
Fluticasone furoate is available over the counter as a nasal spray for the symptomatic treatment of hay fever and other upper respiratory allergies in patients ≥2 years old.
Definition
ChEBI: A trifluorinated corticosteroid that consists of 6alpha,9-difluoro-11beta,17alpha-dihydroxy-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-16-methyl-3-oxoandrosta-1,4-diene bearing a 2-furoyl s bstituent at position 17. Used in combination with vilanterol trifenate for treatment of bronchospasm associated with chronic obstructive pulmonary disease.
brand name
Veramyst
Pharmacokinetics
Fluticasone furoate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Though effective for the treatment of asthma, corticosteroids may not affect symptoms immediately. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Trials in subjects with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone furoate. This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic bioavailability (approximately 1.3%), and the minimal pharmacological activity of the metabolites detected in man.
Inhaled fluticasone furoate at repeat doses of up to 400 mcg in healthy subjects was not associated with statistically significant decreases in serum or urinary cortisol in healthy subjects. Reductions in serum and urine cortisol levels were observed at fluticasone furoate exposures several-fold higher than exposures observed at the therapeutic dose. For subjects with asthma, a randomized, double-blind, parallel-group trial in 104 pediatric subjects showed no difference between once-daily treatment with 50 mcg fluticasone compared with placebo on serum cortisol weighted mean (0 to 24 hours) and serum cortisol AUC(0-24) following 6 weeks of treatment.
A randomized, double-blind, parallel-group trial in 185 subjects with asthma aged 12 to 65 years showed no difference between once-daily treatment with fluticasone furoate/vilanterol 100 mcg/25 mcg or fluticasone furoate/vilanterol 200 mcg/25 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours), serum cortisol AUC(0-24), and 24-hour urinary cortisol after 6 weeks of treatment, whereas prednisolone 10 mg given once daily for 7 days resulted in significant cortisol suppression.
A QT/QTc trial did not demonstrate an effect of fluticasone furoate administration on the QTc interval. The effect of a single dose of 4,000 mcg of orally inhaled fluticasone furoate on the QTc interval was evaluated over 24 hours in 40 healthy male and female subjects in a placebo and positive-controlled (a single dose of 400 mg oral moxifloxacin) cross-over trial. The QTcF maximal mean change from baseline following fluticasone furoate was similar to that observed with placebo with a treatment difference of 0.788 msec (90% CI: -1.802, 3.378). In contrast, moxifloxacin given as a 400-mg tablet resulted in prolongation of the QTcF maximal mean change from baseline compared with placebo with a treatment difference of 9.929 msec (90% CI: 7.339, 12.520).
Synthesis
The synthesis of fluticasone on large scale was disclosed in the patent literature. The starting 6|á ,9|á- difluoro-11|?-17|á-dihydroxy-16|á-methyl-3-oxoandrosta-1,4- diene-17|?-carboxylic acid 35 was converted to the analogous carbothioic acid 36 in 95% yield via activation with carbonyl diimidazole, followed by reaction with hydrogen sulfide gas. Conversion of the carbothioic acid to fluticasone was completed through a three-step sequence in a one pot process in 99% overall yield. Carbothioic acid 36 and DMAP were dissolved in MEK. Tripropylamine (TPA) was then added to the mixture at -8 to -5??C. Neat furoyl chloride was then added dropwise over 2-3 minutes and the resulting mixture was then stirred at -5 to 0??C for 15 minutes generating a mixture of desired ester 37 and thioanhydride 38. A solution of N-methylpiperazine in water was then added dropwise over 2-3 minutes at -5 to 0??C to the crude reaction mixture and stirred for 10 minutes, which enabled the conversion of thioanhydride 38 to the ester 37. A solution of bromofluoromethane in MEK was then added rapidly at 0??C and the resulting solution was stirred at 20??C for 5 hours. After a simple work-up, fluticasone furoate (V) was obtained in 99% overall yield from 36 with 97% purity.
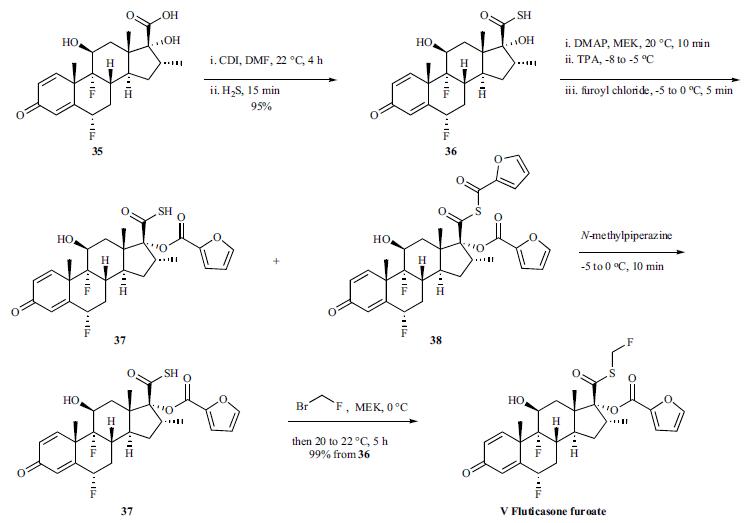
Metabolism
Fluticasone furoate is cleared from systemic circulation principally by hepatic metabolism via CYP3A4 to metabolites with significantly reduced corticosteroid activity. There was no in vivo evidence for cleavage of the furoate moiety resulting in the formation of fluticasone. Fluticasone furoate is also hydrolyzed at the FIVE-S-fluoromethyl carbothioate group, forming an inactive metabolite.
Properties of FLUTICASONE FUROATE
| Melting point: | 250-252°C (dec.) |
| Boiling point: | 625.2±55.0 °C(Predicted) |
| Density | 1.39 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly, Heated, Sonicated), Methanol (Slightly) |
| form | Solid |
| pka | 12.52±0.70(Predicted) |
| color | White to Off-White |
Safety information for FLUTICASONE FUROATE
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for FLUTICASONE FUROATE
FLUTICASONE FUROATE manufacturer
BDR Pharmaceuticals International Pvt Ltd
Symbiotec Pharma Lab Pvt Ltd
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
 397864-44-7 FLUTICASONE FUROATE 99%View Details
397864-44-7 FLUTICASONE FUROATE 99%View Details
397864-44-7 -
 Fluticasone Furoate 98%View Details
Fluticasone Furoate 98%View Details -
 397864-44-7 Fluticasone furoate 98%View Details
397864-44-7 Fluticasone furoate 98%View Details
397864-44-7 -
 Fluticasone Furoate 99%View Details
Fluticasone Furoate 99%View Details -
 Fluticasone furoate 99%View Details
Fluticasone furoate 99%View Details -
 Fluticasone furoate 397864-44-7 98%View Details
Fluticasone furoate 397864-44-7 98%View Details
397864-44-7 -
 Fluticasone furoate CAS 397864-44-7View Details
Fluticasone furoate CAS 397864-44-7View Details
397864-44-7 -
 397864-44-7 99%View Details
397864-44-7 99%View Details
397864-44-7