Fluoromethyl phenyl sulfone
- CAS NO.:20808-12-2
- Empirical Formula: C7H7FO2S
- Molecular Weight: 174.19
- MDL number: MFCD00191650
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-22 13:28:40
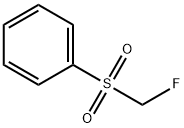
What is Fluoromethyl phenyl sulfone?
Description
Fluoromethyl phenyl sulfone is a useful nucleophilic monofluoromethylation reagent for the synthesis of fluoromethyl alcohols and amines. In the nucleophilic reaction step, strong bases such as LiHMDS and n-BuLi are used to generate the nucleophilic (phenylsulfonyl)fluoromethyl anion. In the desulfonylation step, sodium/mercury amalgam and magnesium are the commonly used reductive reagents. Besides, the addition reaction between fluoromethyl phenyl sulfone and carbonyls can be used to prepare monofluoroaklenes via acylation–elimination.
The Uses of Fluoromethyl phenyl sulfone
Fluoromethyl phenyl sulfone can be used to prepare monofluoroaklenes and chiral α-Monofluoromethyl Amines. Highly stereoselective nucleophilic monofluoromethylation of (R)-(tert-butanesulfinyl)imines with fluoromethyl phenyl sulfone was achieved to afford α-monofluoromethylamines with a nonchelation-controlled stereoselectivity mode. By using the same chemistry, (R)-(tert-butanesulfinyl)imines bearing a terminal tosylate (OTs) group can be converted to α-monofluoromethylated cyclic secondary amines with high stereoselectivity.
Reactions
(1) Monofluoromethylation of aldehydes and ketones.
(2) Monofluoromethylation of aldimines and ketimines.
(3) Monofluoromethylenation of aldehydes and ketones.
(4) (Phenylsulfonyl)fluoromethylation of esters.
References
[1] YA LI. Stereoselective Nucleophilic Monofluoromethylation of N-(tert-Butanesulfinyl)imines with Fluoromethyl Phenyl Sulfone[J]. Organic Letters, 2006. DOI:10.1021/ol060322t.
[2] M. INBASEKARAN. ChemInform Abstract: A NOVEL AND EFFICIENT SYNTHESIS OF FLUOROMETHYL PHENYL SULFONE AND ITS USE AS A FLUOROMETHYL WITTIG EQUIVALENT[J]. ChemInform, 1985. DOI:10.1002/chin.198536160.
[3] GOUVERNEUR V, LOZANO ó. Preparation of Chiral α-Monofluoromethyl Amines Using Fluoromethyl Phenyl Sulfone[C]. 1900. DOI:10.1055/sos-SD-203-00578.
Properties of Fluoromethyl phenyl sulfone
| Melting point: | 53 °C |
| Boiling point: | 151 °C(Press: 0.3 Torr) |
| Density | 1.274±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | soluble in Methanol |
| form | powder to crystal |
| color | White to Almost white |
Safety information for Fluoromethyl phenyl sulfone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Fluoromethyl phenyl sulfone
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Fluoromethyl Phenyl Sulfone CAS 20808-12-2View Details
Fluoromethyl Phenyl Sulfone CAS 20808-12-2View Details
20808-12-2 -
 Fluoromethyl phenyl sulfone 97% CAS 20808-12-2View Details
Fluoromethyl phenyl sulfone 97% CAS 20808-12-2View Details
20808-12-2 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1