Fluazinam
Synonym(s):3-Chloro-N-(3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-2-pyridylamine;3-Chloro-N-(3-chloro-5-trifluoromethyl-2-pyridyl)-α,α,α-trifluoro-2,6-dinitro-p-toluidine;3-Chloro-N-(3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-2-pyridylamine
- CAS NO.:79622-59-6
- Empirical Formula: C13H4Cl2F6N4O4
- Molecular Weight: 465.09
- MDL number: MFCD00214168
- EINECS: 616-712-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
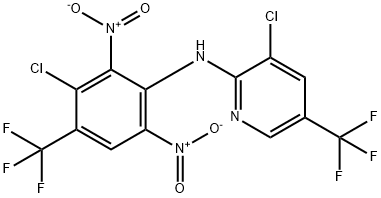
What is Fluazinam?
Description
Fluazinam, with a LogP of 3.56, has very low solubility in water (1.7 mgL?1, pH6.8, 25?C) but higher solubilities in organic solvents (e.g., acetone 470 g/L, dichloromethane 330 g/L, ethanol 150 g/L, all at 20 ?C). The active ingredient forms light yellow crystals with an MP of 115 to 117 ?C and has a vp of 1.5 mPa (25 ?C). The compound is stable to acid, alkali, and heat (5).
Chemical properties
yellow crystal mp 115 ~ 117°C
The Uses of Fluazinam
Fluazinam is a broad spectrum fungicide. Fluazinam is commonly used for control of Sclerotinia blight and other soilborne pathogens of peanut as well for the control of potato blight.
The Uses of Fluazinam
Fluazinam, discovered and developed by ISK, is a highly active fungicide with broad spectrum activity, including soil borne diseases. Furthermore, it has acaricidal activity. Fluazinam is a multi-site contact fungicide that belongs to the pyridinamine family. Fluazinam has launched in several countries since its commercialization in the early 1990's. Now Fluazinam has established a strong presence in potato late blight, soybean white mold or turf fungicide market.
The Uses of Fluazinam
Fluazinam is a fungicide with a protective action but with little curative or systemic activity. It is used to control grey mould and downy mildew on vines, apple scab, southern blight and white mould on peanuts and Phytophthora infestans and tuber blight on potatoes. It is also used against clubroot on crucifers, rhizomania on sugar beet and mites in apples.
Definition
ChEBI: Fluazinam is a member of the class of aminopyridines that is 2-amino-5-(trifluoromethyl)pyridine in which one of the amino hydrogens is replaced by a 3-chloro-2,6-dinitro-4-(trifluoromethyl)phenyl group. A fungicide used to control grey mould, downy mildew and other fungal pathogens. It has a role as an apoptosis inducer, an allergen, a xenobiotic, an environmental contaminant and an antifungal agrochemical. It is a C-nitro compound, a chloropyridine, an aminopyridine, a secondary amino compound, a member of monochlorobenzenes and a member of (trifluoromethyl)benzenes.
General Description
Fluazinam is a 2,6-dinitroaniline protectant fungicide, which belongs to the pyridinamine group. It can mostly find applications in agriculture.
Hazard
Moderately toxic by ingestion and inhalation. A reproductive hazard.
Agricultural Uses
Fungicide: Used to control Sclerotinia blight on peanuts and late blight and white mold on potatoes.
Trade name
FLUAZINAM 50 WP®; FROWNCIDE®; IKF-1216®; ICIA-192®; OMEGA; PP-192®; SHIRLAN®
Contact allergens
Fluazinam is a pesticide with a broad spectrum of anti fungal activity. It caused sensitization in employees in the tulip bulb industry and in farmers. Fluazinam induced contact dermatitis in a worker in a plant where it was manufactured.
Safety Profile
Moderately toxic by ingestion and inhalation. Experimental reproductive effects. When heated to decomposition it emits toxic vapors of NOx, F-, and Cl-.
Metabolic pathway
Fluazinam may typically be applied to crops of potatoes using up to 10 applications each of 150 g ha-1. Routes of degradation involve ring hydroxylation in aerobic soils and reduction of the nitro groups in flooded soils. A large amount of unextractable residue was observed. In potatoes and rats, important reactions involve the reduction of nitro groups and the formation of conjugates. In plants, the trifluoromethyl substituents are hydrolysed to carboxylic acid groups. Most of the information presented below was taken from an evaluation of the fungicide published by the UK Pesticide Safety Directorate (PSD, 1994).
Degradation
Fluazinam is stable to acid, alkali and heat (PM). The DT50 values for hydrolysis in buffer solutions held in the dark at 22 °C were 42 and 5.6 days at pH 7 and 9, respectively. There was no appreciable hydrolysis at pH 5. The major reaction involved hydrolysis of a trifluoromethyl group to give the nicotinic acid derivative (2). The DT50 values of fluazinam in buffer solutions exposed to natural sunlight were in the range 2-3 days. Up to 11 products were separated but only the major product formed at pH 9, the nicotinic acid derivative (2), was characterised (PED, 1994).
Properties of Fluazinam
| Melting point: | 100-102° |
| Boiling point: | 376.1±42.0 °C(Predicted) |
| Density | 1.766±0.06 g/cm3(Predicted) |
| vapor pressure | 1.5 x 10-3 Pa (25 °C) |
| Flash point: | 2 °C |
| storage temp. | 2-8°C |
| solubility | DMF:25.0(Max Conc. mg/mL);53.75(Max Conc. mM) DMSO:25.0(Max Conc. mg/mL);53.75(Max Conc. mM) Ethanol:15.0(Max Conc. mg/mL);32.25(Max Conc. mM) PBS (pH: 7.2):0.25(Max Conc. mg/mL);0.54(Max Conc. mM) |
| form | Solid |
| pka | 7.11(at 25℃) |
| form | neat |
| Water Solubility | 1.7 mg l-1 (25 °C) |
| color | Light yellow to Amber to Dark green |
| Merck | 14,4117 |
| BRN | 4772672 |
| CAS DataBase Reference | 79622-59-6(CAS DataBase Reference) |
| EPA Substance Registry System | Fluazinam (79622-59-6) |
Safety information for Fluazinam
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Fluazinam
| InChIKey | UZCGKGPEKUCDTF-UHFFFAOYSA-N |
Fluazinam manufacturer
Cropnosys India Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
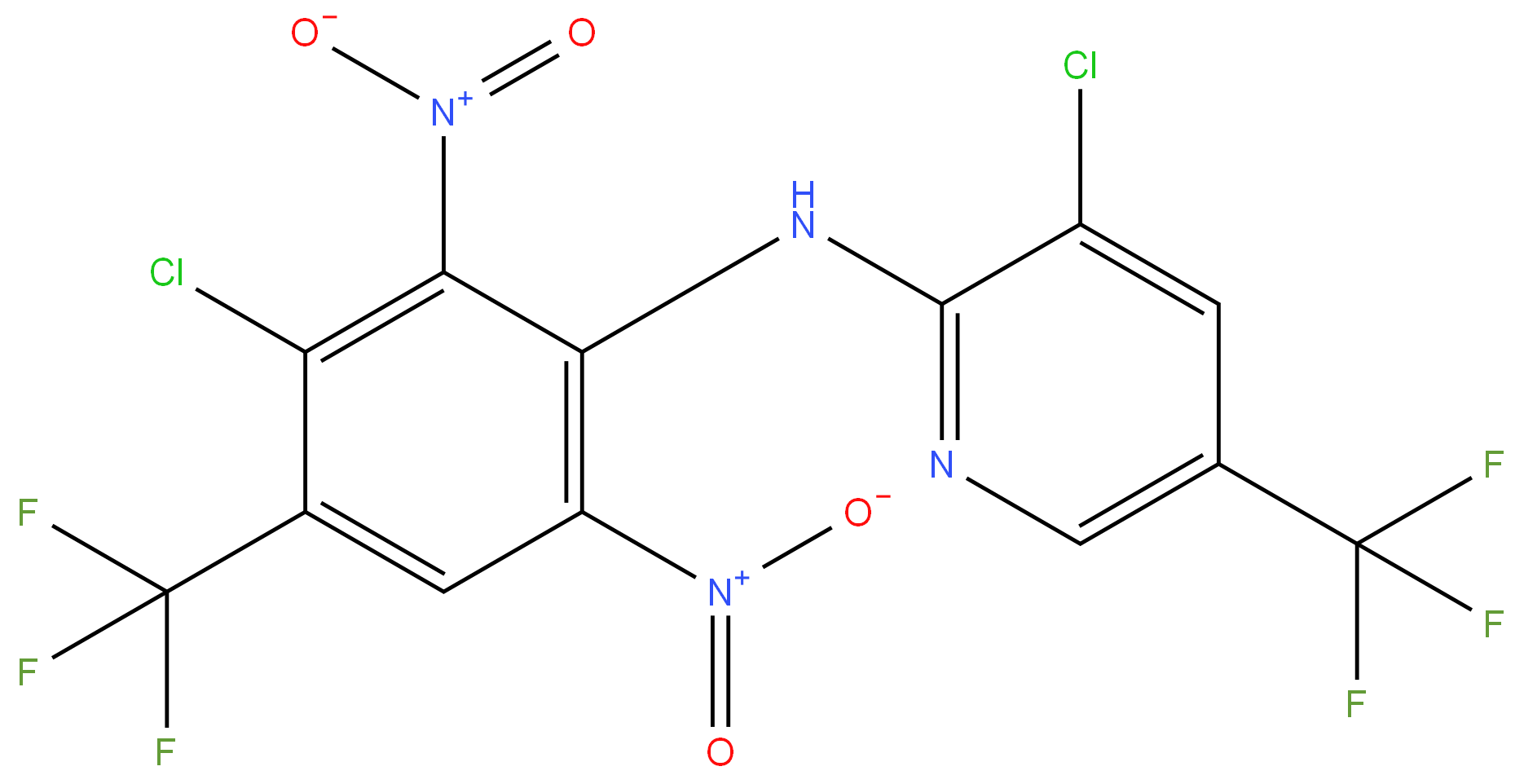 79622-59-6 Fluazinam 99%View Details
79622-59-6 Fluazinam 99%View Details
79622-59-6 -
 Fluazinam CAS 79622-59-6View Details
Fluazinam CAS 79622-59-6View Details
79622-59-6 -
 Fluazinam 98% (HPLC) CAS 79622-59-6View Details
Fluazinam 98% (HPLC) CAS 79622-59-6View Details
79622-59-6 -
 Fluazinam CAS 79622-59-6View Details
Fluazinam CAS 79622-59-6View Details
79622-59-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1