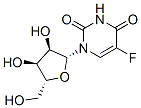
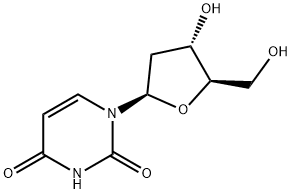
Floxuridine
Synonym(s):floxuridine;FUDR;5-Fluoro-2′-deoxyuridine;FdUrd, FUdR, 5-FdU, Floxuridine;2′-Deoxy-5-fluorouridine
- CAS NO.:50-91-9
- Empirical Formula: C9H11FN2O5
- Molecular Weight: 246.19
- MDL number: MFCD00006530
- EINECS: 200-072-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Floxuridine?
Toxicity
Oral, rat LD50: 215 mg/kg. Signs of overdose include nausea, vomiting, diarrhea, gastrointestinal ulceration and bleeding, and bone marrow depression (including thrombocytopenia, leukopenia and agranulocytosis).
Description
Floxuridine is a nucleoside analog that inhibits the enzyme ribonucleotide reductase, which is involved in the synthesis of DNA. Floxuridine has been shown to inhibit the growth of cancer cells and induce apoptosis in vivo. Floxuridine has also been shown to inhibit tumor growth in animal models by inhibiting the production of reactive oxygen species and upregulating tumor suppressor genes, such as p53. This drug also has inhibitory effects on enzymes that are involved in cell proliferation, such as protein kinase C and tyrosine kinases.
Chemical properties
White Solid
Originator
FUDR,Roche,US ,1971
The Uses of Floxuridine
Floxuridine USP is used in Palliative treatment of gastrointestinal adenocarcinoma with liver metastases.
The Uses of Floxuridine
Antiviral; antineoplastic.
The Uses of Floxuridine
renal function diagnosis
Background
An antineoplastic antimetabolite that is metabolized to fluorouracil when administered by rapid injection. Floxuridine is available as a sterile, nonpyrogenic, lyophilized powder for reconstitution. When administered by slow, continuous, intra-arterial infusion, it is converted to floxuridine monophosphate. It has been used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract.
Indications
For palliative management of gastrointestinal adenocarcinoma metastatic to the liver, when given by continuous regional intra-arterial infusion in carefully selected patients who are considered incurable by surgery or other means. Also for the palliative management of liver cancer (usually administered by hepatic intra-arterial infusion).
What are the applications of Application
5-Fluoro-2′-deoxyuridine is an experimental antiproliferative agent shown to have activity against variety of neoplasms
Definition
ChEBI: A pyrimidine 2'-deoxyribonucleoside compound having 5-fluorouracil as the nucleobase; used to treat hepatic metastases of gastrointestinal adenocarcinomas and for palliation in malignant neoplasms of the liver and gastrointestinal tract.
Manufacturing Process
Cells of Streptococcus fecalis (ATCC-8043) were grown in the AOAC folic acid
assay medium [Lepper, Official and Tentative Methods of the Association of Official Agricultural Chemists, Washington, D.C., 7th edition, 784 (1950)],
supplemented with 2 mg per liter of thymine; following the teachings of
Prusoff, Proc. Soc. Exp. Biol. & Med. 85, 564 (1954). After 20 hours of
incubation at 37°C, the cells were harvested by centrifugation. The collected
cells were washed three times with four volumes of potassium phosphate
buffer solution (M/15 aqueous KH2PO4 solution, adjusted to pH 8.0 by addition
of 2 N aqueous KOH) and the wet cells were weighed. The cells were finally
suspended in the above potassium phosphate buffer solution and ground in a
glass tissue homogenizer.
An amount of enzyme preparation equivalent to 900 mg of wet cells was
made up to 25 ml with the above potassium phosphate buffer solution. 150
mg (1.15 mmol) of 5-fluorouracil and 1.0 gram of thymidine (4.12 mmol)
were dissolved in 15 ml of the above potassium phosphate buffer solution.
The mixture was incubated at 37°C for 18 hours. After this time, enzyme
action was stopped by the addition of four volumes of acetone and one
volume of peroxide-free diethyl ether. The precipitated solids were removed by
filtration, and the filtrate was evaporated under nitrogen at reduced pressure
until substantially all volatile organic solvent had been removed. About 20 ml
of aqueous solution, essentially free of organic solvent, remained. This
solution was diluted to 100 ml with distilled water.
Ten microliters of this solution were submitted to descending chromatography
on a paper buffered with 0.2 N KH2PO4 (pH 7.8), using a solvent mixture of
tertiary amyl alcohol:water:n-butyl ether (80:13:7 by volume). A spot visible
under ultraviolet light and having Rf = 0.55 was leached with 0.1 N HCl and
assayed for deoxyribose by the method of Stumpf, J. Biol. Chem. 169, 367
(1947). This analysis indicated the presence of a minimum of 85.5 mg (0.35
mmol) of 2'-deoxy-5-fluorouridine in the protein-free reaction mixture
according to US Patent 2,885,396. An alternate route from 5-fluorouracil via
the mercury derivative, through toluoyl deoxyuridines and then toluoyl
removal to give floxuridine is described in US Patent 3,041,335.
brand name
Fudr (Mayne).
Therapeutic Function
Antiviral, Cancer chemotherapy
General Description
Inhibits DNA synthesis.
General Description
The drug is available as a 500-mg vial of lyophilized powder.The drug is used to treat metastatic GI adenocarcinoma.The mechanism of action of this fluoropyrimidine deoxynucleosideanalog involves metabolic conversion to 5-fluorouracil(5-FU) metabolites resulting in inhibition of TSthus disrupting DNA synthesis, function, and repair.Resistance can occur because of increased expression of TS,decreased levels of reduced folate 5,10-methylenetetrahydrofolate,increased activity of DNA repair enzymes, and increasedexpression of dihydropyrimidine dehydrogenase(the major catabolic enzyme). The drug is poorly absorbedfrom the GI tract and is extensive metabolized to 5-FU and5-FU metabolites. Dihydropyrimidine dehydrogenase is themain enzyme responsible for 5-FU catabolism, and it ispresent in liver, GI mucosa, white blood cells, and kidney.The drug interaction and toxicity profiles are equivalent tothose of 5-FU.
Health Hazard
ACUTE/CHRONIC HAZARDS: Floxuridine is highly toxic by ingestion.
Fire Hazard
Flash point data for Floxuridine are not available, but Floxuridine is probably combustible.
Biochem/physiol Actions
Antineoplastic drug that acts as a potent inhibitor of thymidylate synthetase Resistance to FUdR can develop in cancer cell cultures, among other means, by low-level Mycoplasma infection.
Pharmacokinetics
Floxuridine is an anti-metabolite or a pyrimidine analog that works by disrupting the process S-phase of cell division, selectively targeting rapidly dividing cells. Due to the structural similarities, antimetabolites act as pyrimidine-like molecules and prevent normal pyrimidines from being incorporated into DNA. After successful biotransformation, floxuridine is converted into an active component, flurouracil, which blocks the enzyme which converts cytosine nucleosides into the deoxy derivative. Flurouracil also physically prevents the incorporation of thymidine nucleotides into the DNA strand by taking their place, further preventing DNA synthesis.
Safety Profile
Poison by ingestion. Moderately toxic by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. Human systemic effects: hypermotitity, diarrhea, nausea, vomiting and other gastrointestinal effects, allergic dermatitis, and bone marrow changes. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Fand NOx.
Synthesis
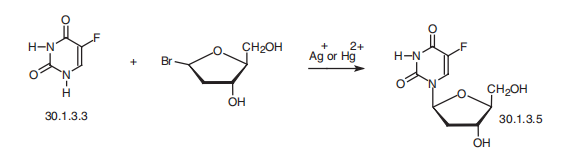
Fluoxuridine, 5-fluoro-1-(2-deoxyribofuranosyl)-pyrimidin-2,4-(1H,3H)- dione (30.1.3.5), is a pyrimidine nucleotide made by reacting fluorouracil (30.1.3.3) with 2-deoxyribofuranosylbromide in the presence of silver or mercury salts.
Metabolism
Hepatic.
Properties of Floxuridine
| Melting point: | 148 °C(lit.) |
| Boiling point: | 150 °C |
| alpha | 35.9 º (c=1, water) |
| Density | 1.3751 (estimate) |
| storage temp. | room temp |
| solubility | Soluble to 100 mM in water and to 100 mM in DMSO |
| form | Powder |
| pka | pKa 7.44 (Uncertain) |
| color | White to almost white |
| Water Solubility | soluble |
| Merck | 14,4112 |
| BRN | 2645818 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-91-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Uridine, 2'-deoxy-5-fluoro-(50-91-9) |
| EPA Substance Registry System | Floxuridine (50-91-9) |
Safety information for Floxuridine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Floxuridine
| InChIKey | ODKNJVUHOIMIIZ-GFCOJPQKSA-N |
Floxuridine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 50-91-9 98%View Details
50-91-9 98%View Details
50-91-9 -
 50-91-9 99%View Details
50-91-9 99%View Details
50-91-9 -
 5-Fluoro-2'-deoxyuridine CAS 50-91-9View Details
5-Fluoro-2'-deoxyuridine CAS 50-91-9View Details
50-91-9 -
 5-Fluoro-2-Deoxyuridine extrapure CAS 50-91-9View Details
5-Fluoro-2-Deoxyuridine extrapure CAS 50-91-9View Details
50-91-9 -
 2'-Deoxy-5-fluorouridine CAS 50-91-9View Details
2'-Deoxy-5-fluorouridine CAS 50-91-9View Details
50-91-9 -
 Floxuridine CAS 50-91-9View Details
Floxuridine CAS 50-91-9View Details
50-91-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
