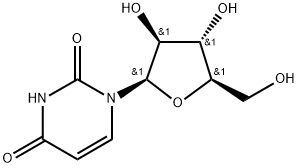
2'-Deoxyuridine
Synonym(s):1-(2-Deoxy-β-D -ribofuranosyl)uracil;Uracil deoxyriboside
- CAS NO.:951-78-0
- Empirical Formula: C9H12N2O5
- Molecular Weight: 228.2
- MDL number: MFCD00006527
- EINECS: 213-455-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
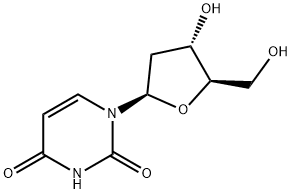
What is 2'-Deoxyuridine?
Description
2'-Deoxyuridine is an intermediate in the synthesis of thymidylate, which is a precursor for DNA synthesis. It has been shown to inhibit the enzymatic activity of enzymes responsible for synthesizing uridine and thymidylate, leading to neuronal death. 2'-Deoxyuridine has been used as a fluorescence probe for nucleic acids and as a polymerase chain reaction (PCR) substrate. It is also known to bind with toll-like receptor 4 (TLR4), which is involved in inflammatory responses.
Chemical properties
White crystalline powder
The Uses of 2'-Deoxyuridine
An uridine derivative as therapeutic agent for treating allergy, cancer, infection and autoimmune disease
The Uses of 2'-Deoxyuridine
2'-Deoxyuridine is frequently halogenated to create thymidine analogues useful for studies of DNA synthesis and degradation mechanisms. Derivatized 2'-Deoxyuridines used as labeling substrates include chloro-2'-deoxyuridine (CldU), bromodeoxyuridine (BrdU) and/or iododeoxyuridine (IdU). Other useful analogues of 2'-deoxyuridine include 5-ethynyl-2'-deoxyuridine (DdU) and 5-hydroxymethyl-2'-deoxyuridine (HmdU). Laboratory suppression of deoxyuridine is used to diagnose megaloblastic anemias due to vitamin B12 and folate deficiencies. Deoxyuridine (dU) is used to indirectly determine if there are sufficient levels of folate and cobalamin in cell or tissue samples.
Definition
ChEBI: 2'-deoxyuridine is a pyrimidine 2'-deoxyribonucleoside having uracil as the nucleobase. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is functionally related to a uracil.
Biological Activity
2'-deoxyuridine is frequently halogenated to create thymidine analogues useful for studies of dna synthesis and degradation mechanisms.
Properties of 2'-Deoxyuridine
| Melting point: | 167-169 °C(lit.) |
| Boiling point: | 370.01°C (rough estimate) |
| alpha | D22 +50° (c = 1.1 in N NaOH) |
| Density | 1.3705 (rough estimate) |
| refractive index | 52 ° (C=1, 1mol/L NaOH) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated), Water (Slightly) |
| form | Powder |
| pka | pKa 9.3(H2O
t = 25) (Uncertain) |
| color | White to off-white |
| Water Solubility | 300 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,2910 |
| BRN | 24433 |
| CAS DataBase Reference | 951-78-0(CAS DataBase Reference) |
| EPA Substance Registry System | Uridine, 2'-deoxy- (951-78-0) |
Safety information for 2'-Deoxyuridine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2'-Deoxyuridine
| InChIKey | MXHRCPNRJAMMIM-ATRFCDNQSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 2'-Deoxyuridine CAS 951-78-0View Details
2'-Deoxyuridine CAS 951-78-0View Details
951-78-0 -
 2’-Deoxyuridine extrapure CAS 951-78-0View Details
2’-Deoxyuridine extrapure CAS 951-78-0View Details
951-78-0 -
 2′-Deoxyuridine CAS 951-78-0View Details
2′-Deoxyuridine CAS 951-78-0View Details
951-78-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
