Fisetin
Synonym(s):3,3′,4′,7-Tetrahydroxyflavone;3,3′,4′,7-Tetra-hydroxy-flavone;5-Deoxyquercetin;5-Desoxyquercetin;Cotinin
- CAS NO.:528-48-3
- Empirical Formula: C15H10O6
- Molecular Weight: 286.24
- MDL number: MFCD00006829
- EINECS: 208-434-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
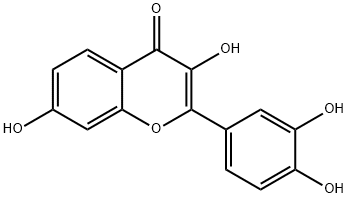
What is Fisetin?
Description
Fisetin, a well-known plant flavonol from natural flavonoid group, is found in traditional medicines, plants, vegetables, fruits. Fisetin also has anti-oxidant, anti-inflammatory, anti-tumor effects[1].
Chemical properties
OCHRE POWDER
The Uses of Fisetin
Fisetin is used in biological studies as spleen tyrosine kinase inhibitors for autoimmune inflammation disease. This compound has neuroprotective properties.
What are the applications of Application
Fisetin is a potent sirtuin activating compound (STAC), antiinflammatory and anticancer agent
Definition
ChEBI: Fisetin is a 7-hydroxyflavonol with additional hydroxy groups at positions 3, 3' and 4'. It has a role as an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an antioxidant, an anti-inflammatory agent, a metabolite, a plant metabolite and a geroprotector. It is a 3'-hydroxyflavonoid, a 7-hydroxyflavonol and a tetrahydroxyflavone. It is a conjugate acid of a fisetin(1-).
What are the applications of Application
Fisetin is primarily used in laboratory bioactivity studies as an analytical standard for qualitative and quantitative analyses of Fisetin, and as a dietary supplement, and sells well for its potential anti-aging and cognitive enhancing effects. Because of its potent antioxidant and anti-inflammatory properties, it has been shown to have varying degrees of inhibitory effects on a wide range of diseases (e.g., cancer, Alzheimer's disease, anti-aging cells, and diabetes), and has been extensively studied in terms of its pharmacological effects in vitro and in vivo.
Benefits
Fisetin is known to have antioxidant properties and demonstrates the specific biological activity of protecting functional macromolecules against stress, resulting in a benefit to cellular cytoprotection.It is also known to have anti-inflammatory, chemopreventive, and chemotherapeutic properties.
General Description
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG
Anticancer Research
Fisetin is a plant polyphenol from the flavonoid group. It occurs in fruits and vegetablesincluding persimmons, strawberries, onions, cucumbers, and apples. It is anantioxidant, exerts anticarcinogenic effects in HCT-116 (human colon carcinoma)cells, and modulates protein kinase and lipid kinase pathways (Wang et al. 2012).Fisetin alter signaling pathway like MAPK, NF-κB, activators of transcription(JAK/STAT), Janus kinase/signal transducers, phosphoinositide-3-kinase-proteinkinase (PI3K/Akt), Wnt, and mammalian target of rapamycin (mTOR), therebyleading to cell cycle arrest in HL-60 cells (human acute promyelocytic leukemiacells) (Singh et al. 2016b). Thus, it exhibits inhibitory effects on adhesion, migration,invasion, and multidrug resistance (Suh et al. 2009).
Side Effects
Fisetin is generally considered safe and no significant toxicity has been reported in humans when consumed in dietary amounts. However, as with any dietary supplement or medication, excessive intake of laccasein supplements may result in adverse reactions. Some of the potential adverse effects of laccasein supplementation may include gastrointestinal disturbances such as nausea, vomiting, and diarrhoea. In addition, fisetin may interact with certain medications, such as blood thinners, and may increase the risk of bleeding. fisetin may also have estrogenic activity, which means that it may interact with the body's endocrine system and affect individuals with hormone-sensitive diseases (e.g., breast cancer); therefore, it is important to take precautions before taking fisetin supplements, especially for individuals with a history of hormone-related medical conditions or who are taking medications that may interact with fisetin.
Source
Fisetin is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers.
storage
Store at -20°C
References
[1] KIM T W. Fisetin, an Anti-Inflammatory Agent, Overcomes Radioresistance by Activating the PERK-ATF4-CHOP Axis in Liver Cancer.[J]. International Journal of Molecular Sciences, 2023, 24 10. DOI:10.3390/ijms24109076.
Properties of Fisetin
| Melting point: | >330 °C(lit.) |
| Boiling point: | 348.61°C (rough estimate) |
| Density | 1.2981 (rough estimate) |
| refractive index | 1.4413 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 6.83±0.40(Predicted) |
| Colour Index | 75620 |
| color | Pale Yellow to Very Dark Yellow |
| Merck | 14,4088 |
| BRN | 292829 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C15H10O6/c16-8-2-3-9-12(6-8)21-15(14(20)13(9)19)7-1-4-10(17)11(18)5-7/h1-6,16-18,20H |
| EPA Substance Registry System | Fisetin (528-48-3) |
Safety information for Fisetin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Fisetin
| InChIKey | XHEFDIBZLJXQHF-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(O)C(O)=C2)OC2=CC(O)=CC=C2C(=O)C=1O |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Fisetin 95% CAS 528-48-3View Details
Fisetin 95% CAS 528-48-3View Details
528-48-3 -
 3,3',4',7-Tetrahydroxyflavone CAS 528-48-3View Details
3,3',4',7-Tetrahydroxyflavone CAS 528-48-3View Details
528-48-3 -
 Fisetin CAS 528-48-3View Details
Fisetin CAS 528-48-3View Details
528-48-3 -
 Fisetin CAS 528-48-3View Details
Fisetin CAS 528-48-3View Details
528-48-3 -
 Fisetin CAS 528-48-3View Details
Fisetin CAS 528-48-3View Details
528-48-3 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8