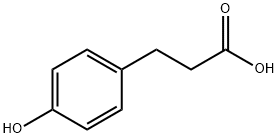
4-Hydroxyphenylacetic acid
- CAS NO.:156-38-7
- Empirical Formula: C8H8O3
- Molecular Weight: 152.15
- MDL number: MFCD00004347
- EINECS: 205-851-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
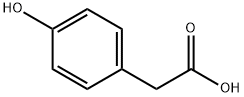
What is 4-Hydroxyphenylacetic acid?
Chemical properties
4-Hydroxyphenylacetic acid is a white to cream or light tan crystalline powder. sublimation. Insoluble in cold water, soluble in hot water, ether, ethanol, ethyl acetate. In the case of ferric chloride, it is a purple-green precipitate. It is used as a pharmaceutical raw material for the synthesis of cephalosporin antibiotics, antipyretic analgesics.
The Uses of 4-Hydroxyphenylacetic acid
4-Hydroxyphenylacetic acid (4-HPAA) is a natural phenolic metabolite and is used as a reagent for the acylation of phenols and amines, as an intermediate in fine chemicals, and as a fluorometric assay for oxidative enzymes. Clinically, 4-HPAA is also used as an ecological disorder marker, and urinary phenylacetic acid has been shown to be negatively correlated with depressive symptoms.
Definition
ChEBI: 4-hydroxyphenylacetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is substituted by a 4-hydroxyphenyl group. It has a role as a plant metabolite, a fungal metabolite, a human metabolite and a mouse metabolite. It is a monocarboxylic acid and a member of phenols. It derives from an acetic acid. It is a conjugate acid of a 4-hydroxyphenylacetate.
Preparation
4-Hydroxyphenylacetic acid is synthesized by diazotization and hydrolysis of 4-aminophenylacetic acid.
4-aminophenylacetic acid and alkali solution are prepared into sodium salt, and then sulfuric acid is added. Cool to 0°C, control the temperature at 0-5°C, and add sodium nitrate solution dropwise, and the reaction is completed for 0.5h. The obtained diazonium was added dropwise to dilute sulfuric acid at 90-95°C for about 1 hour, and the reaction was continued for 1 hour. The reaction solution was decolorized and filtered, cooled and extracted with ethyl acetate, and the extract was recovered with ethyl acetate to obtain 4-Hydroxyphenylacetic acid. The yield is about 85%.
Purification Methods
Crystallise the acid from water or Et2O/pet ether. The p-bromophenacyl ester has m 117o (from EtOH). [Beilstein 10 II 112, 10 III 430, 10 IV 543.]
Properties of 4-Hydroxyphenylacetic acid
| Melting point: | 148-151 °C(lit.) |
| Boiling point: | 234.6°C (rough estimate) |
| Density | 1.2143 (rough estimate) |
| vapor pressure | 0.007Pa at 25℃ |
| refractive index | 1.4945 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 50g/l |
| pka | 4.50±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to cream or light tan |
| PH | 2.0-2.4 (30g/l, H2O, 20℃) |
| Water Solubility | Soluble in dimethyl sulfoxide and methanol. Slightly soluble in water. |
| BRN | 1448766 |
| CAS DataBase Reference | 156-38-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetic acid, 4-hydroxy-(156-38-7) |
| EPA Substance Registry System | Benzeneacetic acid, 4-hydroxy- (156-38-7) |
Safety information for 4-Hydroxyphenylacetic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Hydroxyphenylacetic acid
| InChIKey | XQXPVVBIMDBYFF-UHFFFAOYSA-N |
4-Hydroxyphenylacetic acid manufacturer
JSK Chemicals
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran


You may like
-
 156-38-7 4-hydroxy Phenyl Acetic Acid 99%View Details
156-38-7 4-hydroxy Phenyl Acetic Acid 99%View Details
156-38-7 -
 156-38-7 99%View Details
156-38-7 99%View Details
156-38-7 -
 4-Hydroxyphenylacetic acid 156-38-7 98%View Details
4-Hydroxyphenylacetic acid 156-38-7 98%View Details
156-38-7 -
 156-38-7 4-Hydroxy Phenyl acetic acid 99%View Details
156-38-7 4-Hydroxy Phenyl acetic acid 99%View Details
156-38-7 -
 4-Hydroxyphenylacetic acid 98%View Details
4-Hydroxyphenylacetic acid 98%View Details -
 156-38-7 4-Hydroxyphenylacetic acid, 98% 99%View Details
156-38-7 4-Hydroxyphenylacetic acid, 98% 99%View Details
156-38-7 -
 4-Hydroxyphenylacetic acid, 97%+ CAS 156-38-7View Details
4-Hydroxyphenylacetic acid, 97%+ CAS 156-38-7View Details
156-38-7 -
 4-Hydroxyphenylacetic Acid CAS 156-38-7View Details
4-Hydroxyphenylacetic Acid CAS 156-38-7View Details
156-38-7
