Meprobamate
Synonym(s):2-Methyl-2-propylpropane-1,3-diol dicarbamate;MPB
- CAS NO.:57-53-4
- Empirical Formula: C9H18N2O4
- Molecular Weight: 218.25
- MDL number: MFCD00063428
- EINECS: 200-337-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
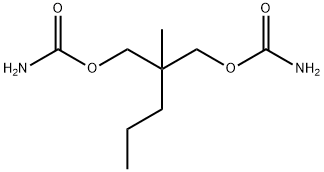
What is Meprobamate?
Absorption
Well absorbed from the gastrointestinal tract.
Toxicity
Symptoms of overdose include coma, drowsiness, loss of muscle control, severely impaired breathing, shock, sluggishness, and unresponsiveness. There have been a case of death resulting from oral ingestion of 12 g of meprobamate. One case study reports the consumption of 40 g of meprobamate resulting in successful survival.
Chemical properties
white crystalline powder
Originator
Equanil,Wyeth,US,1955
The Uses of Meprobamate
An anxiolytic. This is a controlled substance.
Indications
For the management of anxiety disorders or for the short-term relief of the symptoms of anxiety.
Background
A carbamate with hypnotic, sedative, and some muscle relaxant properties, although in therapeutic doses reduction of anxiety rather than a direct effect may be responsible for muscle relaxation. Meprobamate has been reported to have anticonvulsant actions against petit mal seizures, but not against grand mal seizures (which may be exacerbated). It is used in the treatment of anxiety disorders, and also for the short-term management of insomnia but has largely been superseded by the benzodiazepines. (From Martindale, The Extra Pharmacopoeia, 30th ed, p603) Meprobamate is a controlled substance in the U.S.
Definition
ChEBI: Meprobamate is an organic molecular entity.
Manufacturing Process
A solution containing 52.8 parts of 2-methyl-2-n-propyl-1,3-propanediol and 128 parts of acetone is added with stirring to 112 parts of liquid phosgene at such a rate that the temperature of the reaction is maintained at -5° to 0°C. The reaction is stirred one hour at about 0°C then cooled to -15°C. A cooled 30% solution of 32 parts of sodium hydroxide is added with stirring to the reaction at such a rate that the temperature is maintained at -15° to -5°C. The mixture is stirred for an additional ? hour at about 0°C then cooled to - 20°C. 180 parts of cooled ammonium hydroxide solution (28.6% NH3) are added while cooling and with stirring at such a rate that the temperature rises slowly to 20°C and stirring is continued for an additional ? hour. The mixture is poured with agitation into 1,700 parts of ice water. The solid which separates is removed by filtration and dried. Recrystallization from water gives 55 parts (63% of theoretical yield) of 2-methyl-2-n-propyl-1,3-propanediol dicarbamate, MP 104° to 105°C.
brand name
Amosene (Ferndale); Bamate (Alra); Equanil (Wyeth); Mepriam (Teva); Meprospan (Medpointe); Miltown (Medpointe); Neuramate (Halsey); Tranmep (Solvay Pharmaceuticals);3p bamte;Apo-meprobamate;Carb-a-med;Clindoorm;Cusitan;Daritran;Detensitral;Dolovisano;Dormilfo n;Dystoid;Edental;Equiner;Equqtrqte;Gene-bamate;Idemin;Indemin;Irs 109 a;Iterco;Juvamidon;Lan-dol;Lenicor;M.a.s.;M.p. trantabs;Margaris;Meditran;Mep-e;Meprobadal;Meprobil;Meprogesic q;Meprolin;Mepronel;Mepro-secergan 400;Meproserpina;Meprospan 400;Meproten;Meproyrin;Meprozine;Meriprobate;Microbamat;Midixin;Milspan;Miltown s-r;Neo-nervostal;Neo-tran;Neurocalm;Novomato;Novomepro;Nyktogen;Oasil procalmadiol;Odsil 10;Panquil;Pensive;Pentaneural;Pm 2;Pmb 4000;Prequil;Probal;Probasan;Probromato;Psico-retard;Quietidon;Regium;Seda baxacor;Sedavier;Selene;Selodorm;Serenade;Sintown;Sopanil;Spasmobamat;Tcm 200;Tcm 400;Trankilin;Trelmar;Tri-reumo-campil;Vasocalm;Vistabamate;Wescomep.
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Meprobamate, a bis-carbamate ester, was introduced in 1955 for the treatment of anxiety and was subsequently used as a sedative-hypnotic drug. Psychological and physical dependence can occur and abuse has been reported. Meprobamate is controlled under Schedule IV of the 1971 Convention on Psychotropic Substances. (Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV), , , 1971)
General Description
Odorless white crystalline powder. Bitter taste. Solutions in water are neutral or slightly acidic.
General Description
Meprobamate,2-methyl-2-propyltrimethylene dicarbamate, 2-methyl-2-propyl-1,3-propanediol dicarbamate (Equanil, Miltown), is officiallyindicated as an antianxiety agent. It is also a sedative–hypnotic agent. It has several overall pharmacological properties resembling those of benzodiazepines and barbiturates.The mechanism of action underlying anxiolytic effectsis unknown but may involve effects on conductivity inspecific brain areas.It does not appear to act through effectson GABAergic systems.The drug is effective againstabsence seizures and may worsen generalized tonic–clonicseizures.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Meprobamate is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Hazard
Central nervous system depressant, use restricted by law.
Fire Hazard
Flash point data for Meprobamate are not available; however, Meprobamate is probably combustible.
Pharmacokinetics
Meprobamate is an anxiolytic drug. It was the best selling minor tranquilizer for a time but has largely been replaced by benzodiazepines. Meprobamate has most of the pharmacological effects and dangers of the barbiturates (though it was marketed as being safer) but it is less sedating at effective doses. Meprobamate exhibits some anticonvulsant effects in absence seizures; however, it is reported to potentially exacerbate generalized tonic-clonic seizures. It has also been used as a hypnotic (sleeping pill). However, its is currently only licensed as an anxiolytic and it is a third or fourth-order choice.
Clinical Use
Meprobamate is also a centrally acting skeletal musclerelaxant. The agents in this group find use in several conditions,such as strains and sprains that may produce acutemuscle spasm. They have interneuronal blocking propertiesat the level of the spinal cord, which are said to bepartly responsible for skeletal muscle relaxation.Also,the general CNS depressant properties they possess maycontribute to, or be mainly responsible for, the skeletalmuscle relaxant activity.
Safety Profile
Human poison by unspecified routes. Moderately toxic to humans and experimentally by ingestion. Experimental poison by intravenous, intraperitoneal, and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: coma, blood pressure decrease, regional or general arteriolar constriction, dyspnea, cyanosis, respiratory depression, nausea or vomiting, and allergic skin dermatitis. Experimental reproductive effects. Mutation data reported. Implicated in aplastic anemia. Used as a tranquilizer. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Meprobamate undergoes hepatic metabolism.
Purification Methods
Crystallise it from hot water, aqueous EtOH (m 104-105.5o) or xylene (m 104.1-105.3o). It can be an addictive drug. [Beilstein 3 IV 73.]
Properties of Meprobamate
| Melting point: | 97-100°C |
| Boiling point: | 358.93°C (rough estimate) |
| Density | 1.2004 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 50 mg/ml; DMF:PBS(pH 7.2)(1:1): 0.5 mg/ml; DMSO: 30 mg/ml; Ethanol: 25 mg/ml |
| pka | 13.09±0.50(Predicted) |
| form | neat |
| Water Solubility | 6.2g/L(25 ºC) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-53-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Propanediol, 2-methyl-2-propyl-, dicarbamate(57-53-4) |
| EPA Substance Registry System | Meprobamate (57-53-4) |
Safety information for Meprobamate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Meprobamate
Meprobamate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
