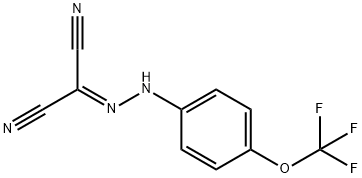
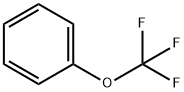
FCCP
Synonym(s):FCCP;Mesoxalonitrile 4-trifluoromethoxyphenylhydrazone
- CAS NO.:370-86-5
- Empirical Formula: C10H5F3N4O
- Molecular Weight: 254.17
- MDL number: MFCD00009699
- EINECS: 206-730-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is FCCP?
Description
FCCP (370-86-5) is an extremely potent uncoupler of mitochondrial oxidative phosphorylation (IC50 = 20 nM).1 Induces apoptosis in a variety of cell lines.2 Inhibits wild type and mutant beta-amyloid production in embryonic kidney 293 cells expressing either wild-type or “Swedish” mutant APP.3 An important tool for inhibiting mitochondrial membrane potential.2,4 Cell permeable.
The Uses of FCCP
FCCP is a potent uncoupler of oxidative phosphorylation in mitochondria that disrupts ATP synthesis by transporting protons across cell membranes. At 40 μM, FCCP induces complete depolymerization of microtubules by increasing intracellular pH via the disruption of the mitochondrial H+ gradient and by decreasing the stability of microtubules by impairing the binding of microtubule-associated proteins.[Cayman Chemical]
What are the applications of Application
FCCP is a very potent oxidative phosphorylation uncoupler found in mitochondria.
Definition
ChEBI: A hydrazone that is hydrazonomalononitrile in which one of the hydrazine hydrogens is substituted by a p-trifluoromethoxyphenyl group.
Biological Activity
A very potent uncoupler of oxidative phosphorylation in mitochondria.
Biochem/physiol Actions
FCCP is a protonophore (H+ ionophore) and uncoupler of oxidative phosphorylation in mitochondria. It is capable of depolarizing plasma and mitochondrial membranes. FCCP has been shown to have a number of effects on cellular calcium. It also is reported to inhibit a background K+ current and induce a small inward current, reduce pH by 0.1 unit, and induce a rise of intracellular [Na+]. FCCP stimulates Mg2+-ATPase activity, inhibits β-amyloid production, and mimics the effect of selective glutamate agonist N-methyl-D-aspartate (NMDA) on mitochondrial superoxide production.
in vitro
fccp increases the rate of cellular o2 consumption. in pc-3 and du-145 prostate cancer cells, the compound could significantly decrease hypoxic as well as normoxic hif-1 transcriptional activity which was in part mediated by down-regulation of the oxygen labile hif-1α and hif-2α protein levels. during hypoxic as well as normoxic, it decreases the expression of hif target genes, vegf and vegf receptor-2. [2]
in vivo
fccp reduces mitochondrial membrane potential and atp production in 8-cell mouse embryos. also, the number of inner cell mass cells decrease within blastocysts with unchanged blastocyst development. this perturbed embryonic mitochondrial function is concomitant with reduced birth weight in female offspring following embryo transfer, which persists until weaning. although fccp-treated males also exhibits reduced glucose tolerance as female, their insulin sensitivity and adiposity gain between 4 and 14 weeks is unchanged. reducing mitochondrial function and, thus, decreasing atp output in the precompacting embryo can influence offspring phenotype. [3]
Storage
Store at RT
References
1) Benz R & McLaughlin S, (1983). The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone).; Biophys J 41 381 2) Gautier et al. (2000), A moderate but not total decrease of mitochondrial membrane potential triggers apoptosis in neuron-like cells.; Neuroreport 11 2953 3) Connop et al. (1999), Novel effects of FCCP [carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone] on amyloid precursor protein processing; J. Neurochem. 72 1457 4) Collins et al. (2000), Inositol 1,4,5-trisphosphate-induced Ca2+ release in inhibited by mitochondrial depolarization.; Biochem. J. 347 593
Properties of FCCP
| Melting point: | 174-175 °C (dec.)(lit.) |
| Boiling point: | 293.3±50.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | acetone: 20 mg/mL, clear, very deep greenish yellow |
| form | powder |
| pka | 5.91±0.10(Predicted) |
| color | yellow |
| BRN | 664446 |
| Stability: | Stable for 2 years from date of purchase as supplied. Protect from exposure to moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 370-86-5 |
Safety information for FCCP
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for FCCP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Fccp 95% CAS 370-86-5View Details
Fccp 95% CAS 370-86-5View Details
370-86-5 -
 Carbonyl Cyanide 4-(Trifluoromethoxy)phenylhydrazone CAS 370-86-5View Details
Carbonyl Cyanide 4-(Trifluoromethoxy)phenylhydrazone CAS 370-86-5View Details
370-86-5 -
 Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, ≥98% (TLC) powder CAS 370-86-5View Details
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, ≥98% (TLC) powder CAS 370-86-5View Details
370-86-5 -
 Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone CAS 370-86-5View Details
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone CAS 370-86-5View Details
370-86-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
