CITRININ
Synonym(s):CIT;Citron protein;CTRO;Rho-interacting;(3R,4S)-8-Hydroxy-3,4,5-Trimethyl-6-Oxo-4,6-Dihydro-3H-Isochromene-7-Carboxylic Acid
- CAS NO.:518-75-2
- Empirical Formula: C13H14O5
- Molecular Weight: 250.25
- MDL number: MFCD00006912
- EINECS: 208-257-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 19:11:34
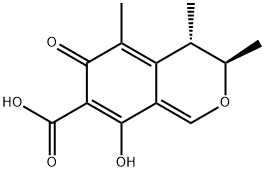
What is CITRININ?
Description
Citrinin is a mycotoxin that has been found in Monascus and has diverse biological activities. It is active against S. aureus, methicillin-resistant S. aureus (MRSA), rifampicin-resistant S. aureus, and vancomycin-resistant E. faecium (MICs = 1.95, 3.9, 0.97, and 7.81 μg/ml, respectively), as well as the pathogenic yeast C. neoformans (MIC = 3.9 μg/ml). It is cytotoxic to a variety of cells in vitro, including bovine kidney cells and mice embryonic stem cells. Citrinin (30 μM) induces reactive oxygen species (ROS) production, mitochondrial membrane potential loss, and apoptosis in HepG2 cells, effects that can be blocked by the antioxidant resveratrol. In contrast, citrinin reduces glutamate-induced excitotoxicity in primary rat cortical neurons at concentrations ranging from 0.1 to 1,000 nM and inhibits LPS-induced production of nitric oxide (NO) in RAW 264.7 cells at 0.625 to 40 μM. It is toxic to brine shrimp larvae (LD50 = 96 μg/ml), as well as to rats and mice with oral LD50 values of 50 and 87-105 mg/kg, respectively. It induces reproductive abnormalities in male mice and toxic effects in the liver, kidney, heart, and gastrointestinal tracts of various animals. Citrinin has been found in stored cereal grains, as well as beans, fruit, and herbs.
Chemical properties
yellow crystalline powder or flakes
Chemical properties
Antimycin (A3C26H36N2O9) and (Antimycin A1) C28H40N2O9 are crystalline solids.
The Uses of CITRININ
Citrinin is a quinonemethine mycotoxin produced by diverse fungi including Aspergillus and Penicillium. Citrinin has been extensively investigate, and is a potent nephrotoxin with hepatoxic and teratogenic activity. Citrinin is the causative agent of Balkan nephropathy and yellow rice fever in humans. At the molecular level, citrinin exhibits a range of effects including free radical damage to DNA and disruption of mitochondrial membrane-bound enzymic activities and structural integrity. Specifically, citrinin is an inhibitor of NADH dehydrogenase in the mitochondrial electron transport chain and this action is responsible for recent reports of citrinin's apoptotic activity.
The Uses of CITRININ
Antibiotic substance produced by a white spore aspergilus which has been placed under the species name Aspergillus niveus. Also produced in small quantities by Penicillium citrinum.
The Uses of CITRININ
antibacterial
What are the applications of Application
Citrinin is an antibiotic which induces mitochondrial permeability pore opening
Definition
ChEBI: (-)-citrinin is a citrinin. It has a role as a Penicillium metabolite. It is an enantiomer of a (+)-citrinin.
General Description
Citrinin is a hepato-nephrotoxic mycotoxin. It can be produced by several filamentous fungi genera of Monauscus, Aspergillus, and Penicillium, which is commonly found as a contaminant in stored grains, foods, feedstuffs and also in biological fluids.
Potential Exposure
Specific uses for antimycin A were not found, however, antimycin A1, and antimycin A3 are reported to be antibiotic substances produced by streptomyces for use as a fungicide, possible insecticide and miticide. Registered as a pesticide in the U.S.
in vitro
previous study found that exposure of hek293 cells to citrinin led to an arrest of cell cycle g2/m in a concentration-dependent increase. citrinin treatment could also elevate the expression levels of p53 and p21 proteins, yet attenuate the signals of phosphorylated cell division cycle 2. moreover, treating hek293 with citrinin could increase both the value of mitotic index and the population of cells, indicating that arrest of citrinin -induced cell cycle occurred mainly during the mitotic phase [1].
in vivo
previous study showed that citrinin acted as a nephrotoxin in all tested animal species, but its acute toxicity varied in different species. the 50% lethal dose for ducks is 57 mg/kg; for chickens it is 95 mg/kg; and for rabbits it is 134 mg/kg. in murine kidneys, citrinin could also synergistically act with ochratoxin a to depress rna synthesis [2].
Storage
+4°C
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
References
[1] chang ch, yu fy, wu ts, wang lt, liu bh. mycotoxin citrinin induced cell cycle g2/m arrest and numerical chromosomal aberration associated with disruption of microtubule formation in human cells. toxicol sci. 2011 jan;119(1):84-92.
[2] j. w. bennett and m. klich. mycotoxins. clin.microbiol.rev. 16(3), 497-516 (2003).
Properties of CITRININ
| Melting point: | 175°C (dec.) |
| Boiling point: | 313.38°C (rough estimate) |
| alpha | D18 -37.4° (c = 1.15 in alc.) |
| Density | 1.37 |
| refractive index | 1.4560 (estimate) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | ≤2mg/ml in ethanol;20mg/ml in DMSO;20mg/ml in dimethyl formamide |
| form | Crystalline Powder or Flakes |
| pka | 1.52±0.60(Predicted) |
| color | Yellow |
| Merck | 13,2351 |
| BRN | 5282243 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 518-75-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 40, Sup 7) 1987 |
| EPA Substance Registry System | Citrinin (518-75-2) |
Safety information for CITRININ
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for CITRININ
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Citrinin solution CAS 518-75-2View Details
Citrinin solution CAS 518-75-2View Details
518-75-2 -
 Citrinin CAS 518-75-2View Details
Citrinin CAS 518-75-2View Details
518-75-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
