CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
Synonym(s):m-Cl-CCP;Carbonyl Cyanide m-Chlorophenylhydrazone - CAS 555-60-2 - Calbiochem;CCCP;Mesoxalonitrile 3-chlorophenylhydrazone
- CAS NO.:555-60-2
- Empirical Formula: C9H5ClN4
- Molecular Weight: 204.62
- MDL number: MFCD00001848
- EINECS: 209-103-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
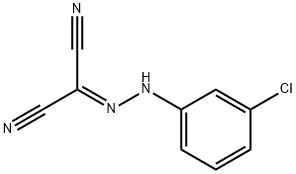
What is CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE?
Description
CCCP is a protonophore mitochondrial uncoupler that increases membrane permeability to protons, leading to a disruption in the mitochondrial membrane potential. It inhibits mitochondrial respiration and ATPase activity when used at concentrations of 110 and 35 nM, respectively. CCCP inhibits activation of stimulation of interferon genes (STING), decreasing the expression of downstream STING targets, including IFN-β, TBK1, and IRF3 when used at a concentration of 50 μM in RAW 264.7 cells. It induces mitochondrial fission in a manner dependent on the GTPase Drp1. CCCP (4 mg/kg) increases body temperature as well as left ventricular systolic and diastolic dimensions and decreases fractional shortening in rats. It also increases myocardial glucose and fatty acid uptake in rats.
Chemical properties
yellow to orange-brown crystalline powder
The Uses of CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
Carbonyl cyanide 3-chlorophenylhydrazone is a widely used uncoupler of oxidative phosphorylation.
The Uses of CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) has been used:
- in the inhibition of efflux pump in clinical Pseudomonas aeruginosa
- to inhibit oxidative phosphorylation activity of mitochondria in human Beas-2B bronchial epithelial cells
- to prepare carbonyl cyanide m-chlorophenylhydrazone solution for oxidative and mitochondrial stress assay and mitophagy induction in Caenorhabditis elegans
What are the applications of Application
Carbonyl Cyanide m-Chlorophenylhydrazone is A proton ionophore commonly used as an uncoupling agent and inhibitor of photosynthesis
Definition
ChEBI: CCCP is a member of the class of monochlorobenzenes that is benzene substituted by 2-(1,3-dinitrilopropan-2-ylidene)hydrazinyl and chloro groups at positions 1 and 3, respectively. It is a mitochondrial depolarizing agent that induces reactive oxygen species mediated cell death. It has a role as a geroprotector, an antibacterial agent and an ionophore. It is a nitrile, a hydrazone and a member of monochlorobenzenes. It is functionally related to a hydrazonomalononitrile.
Biological Activity
Widely used uncoupler of oxidative phosphorylation.
Biochem/physiol Actions
Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) induces mitophagy in mammalian cells. CCCP suppresses the expression of few electron transport chain (ETC) proteins. Protonophore (H+ ionophore) and uncoupler of oxidative phosphorylation in mitochondria. Shown to have a number of effects on cellular calcium. Inhibits secretion of hepatic lipase and partially inhibits the pH gradient-activated Cl- uptake and Cl-/Cl- exchange activities in brush-border membrane vesicles.
in vitro
a genetic β-galactoside reporter system with a disk diffusion assay on macconkey lactose agar petri plates to monitor maintenance of the bacteriophage λ prophage state and viral induction in escherichia coli k-12. it presented evidence that the phage λ major lytic promoters, pl and pr, are activated via cells containing the reporters following exposure to the energy poison cccp. requirement of expression of the λ lytic promoters in response to cccp is host reca function and an auto-cleavable ci repressor, as does sos induction of the λ prophage occurring by a dna damage-dependent pathway. cccp-mediated activation of the λ lytic promoters required λ cro function. cccp does not modulate an sfi-lacz sos reporter [1]. the uncoupler cccp blocks oxidative phosphorylation by damaging the proton gradient. an anion cccp can bind a proton. but cccp with a delocalized negative charge allows it to cross the lipid bilayer in the unprotonated form, while weak acids cross the hydrophobic membrane only when protonated. many protons can be transported by one molecule of cccp across the inner membrane. it is described that a genetic reporter system to monitor maintenance of the λ prophage state and viral induction, and evidence is presented that it activates the major leftward and rightward lytic promoters of the λ prophage that cells are exposed to the energy poison cccp.
storage
Store at RT
References
[1]. thomason lc, court dl. evidence that bacteriophage λ lysogens may induce in response to the proton motive force uncoupler cccp. fems microbiol lett. 2016 feb;363(3).
Properties of CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
| Melting point: | 170-175 °C (dec.) |
| Boiling point: | 333.84°C (rough estimate) |
| Density | 1.3807 (rough estimate) |
| refractive index | 1.6110 (estimate) |
| storage temp. | -20°C |
| solubility | methanol: 10 mg/mL, clear, very deep yellow |
| form | powder |
| pka | 6.00±0.10(Predicted) |
| color | yellow to orange |
| Water Solubility | Insoluble in water. Soluble in DMSO (5 mg/ml), ethanol (1 mg/ml) and methanol (10 mg/ml). |
| BRN | 1842102 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| CAS DataBase Reference | 555-60-2(CAS DataBase Reference) |
| EPA Substance Registry System | Propanedinitrile, [(3-chlorophenyl)hydrazono]- (555-60-2) |
Safety information for CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CARBONYL CYANIDE 3-CHLOROPHENYLHYDRAZONE
| InChIKey | UGTJLJZQQFGTJD-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2-[2-(3,5-DICHLOROPHENYL)HYDRAZONO]MALONONITRILE](https://img.chemicalbook.in/CAS/GIF/3780-83-4.gif)
You may like
-
 Carbonyl cyanide 3-chlorophenylhydrazone CAS 555-60-2View Details
Carbonyl cyanide 3-chlorophenylhydrazone CAS 555-60-2View Details
555-60-2 -
 Carbonyl Cyanide m-Chlorophenylhydrazone CAS 555-60-2View Details
Carbonyl Cyanide m-Chlorophenylhydrazone CAS 555-60-2View Details
555-60-2 -
 Carbonyl cyanide 3-chlorophenylhydrazone CAS 555-60-2View Details
Carbonyl cyanide 3-chlorophenylhydrazone CAS 555-60-2View Details
555-60-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8